Description
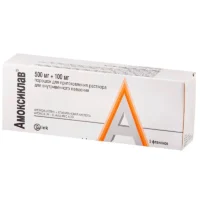
Amoxil (Amoxicillin) Coated Tablets 500 mg/125 mg. №14
Composition
Each coated tablet contains 500 mg of amoxicillin and 125 mg of inactive ingredients.
Mechanism of Action
Amoxicillin, a semisynthetic antibiotic, inhibits bacterial cell wall synthesis by binding to penicillin-binding proteins, thereby disrupting the bacterial cell wall structure and leading to cell lysis.
Pharmacological Properties
Amoxicillin exhibits bactericidal activity against a wide range of Gram-positive and Gram-negative bacteria. It is well-absorbed orally and reaches therapeutic concentrations in various tissues and body fluids.
Indications for Use
Amoxil coated tablets are indicated for the treatment of bacterial infections, including respiratory tract infections, urinary tract infections, skin infections, and other susceptible conditions caused by susceptible organisms.
Contraindications
Avoid using Amoxil if you have a known hypersensitivity to amoxicillin, penicillins, cephalosporins, or any component of the formulation. Use caution in patients with a history of allergic reactions to beta-lactam antibiotics.
Side Effects
Common side effects of Amoxil may include gastrointestinal disturbances, allergic reactions (such as rash or itching), and rarely, severe hypersensitivity reactions like anaphylaxis. Prolonged use may lead to the development of superinfections.
Usage Instructions
The typical adult dosage is 500 mg every 8 hours or 875 mg every 12 hours, depending on the severity of the infection. Dosage adjustments may be necessary in patients with renal impairment. Take the medication as directed by your healthcare provider.
Benefits Compared to Analogues
Amoxil offers broad-spectrum coverage against various bacterial pathogens and has a well-established safety profile. Compared to other antibiotics, it is often preferred for its efficacy, tolerability, and low incidence of serious adverse effects.
Suitable Patient Groups
Amoxil can be used in pediatric and adult populations, including children and the elderly, under appropriate medical supervision. Dosing adjustments may be required based on age, weight, and renal function.
Storage and Shelf Life
Store Amoxil in a cool, dry place away from direct sunlight and moisture. Keep the tablets in their original packaging to protect them from degradation. Check the expiration date on the packaging and do not use expired medication.
Packaging Description
Amoxil tablets are supplied in blister packs containing 14 coated tablets. The packaging is designed to maintain the stability and integrity of the tablets throughout the shelf life.
Clinical Evidence and Proven Effectiveness
Amoxicillin, the active component of Amoxil, has been extensively studied in clinical trials and real-world settings. Clinical studies have demonstrated the efficacy of amoxicillin in treating various bacterial infections, supporting its role as a first-line antibiotic in many clinical guidelines.