Description
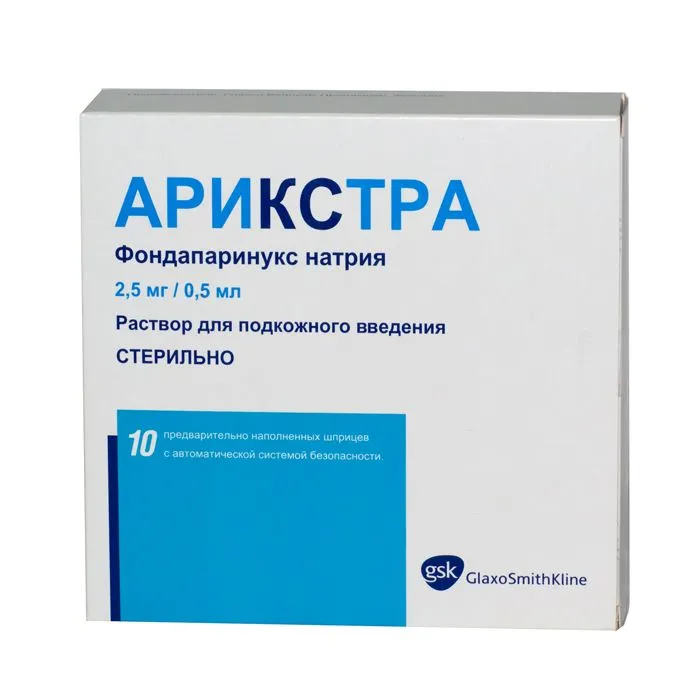
Arixtra (Fondaparinux Natrium) 2.5 mg/0.5 ml. №10
Ingredients
Active ingredient: Fondaparinux Natrium
Inactive ingredients: Sodium chloride, water for injection
Mechanism of Action
Pharmacological Properties: Fondaparinux Natrium, a synthetic pentasaccharide, selectively inhibits factor Xa, effectively preventing blood clot formation.
Indications for Use
Indications: Arixtra is indicated for the prophylaxis of deep vein thrombosis, which may lead to pulmonary embolism, in patients undergoing hip fracture surgery.
Contraindications
Contraindications: Arixtra is contraindicated in patients with active major bleeding, bacterial endocarditis, or severe renal impairment.
Side Effects
Common side effects of Arixtra may include bleeding, bruising, and injection site reactions. Serious side effects may include allergic reactions and thrombocytopenia.
Usage Instructions
Dosage: The usual dose of Arixtra is 2.5 mg administered subcutaneously once daily. Dosage adjustments may be necessary based on individual patient characteristics.
Administration: Arixtra should be administered subcutaneously. It is important to rotate the injection sites and avoid intramuscular administration.
Benefits Compared to Analogues
Arixtra has shown superior efficacy in preventing venous thromboembolism compared to enoxaparin in patients undergoing major orthopedic surgery, as demonstrated in clinical trials.
Suitable Patient Groups
Arixtra is suitable for adult patients undergoing hip fracture surgery for the prophylaxis of deep vein thrombosis. Dosage adjustments are required for patients with renal impairment.
Storage and Packaging
Arixtra should be stored at controlled room temperature. The shelf life of the product is as indicated on the packaging.
Packaging: Arixtra is available in packs containing 10 prefilled syringes of 2.5 mg/0.5 ml.
Clinical Evidence and Effectiveness
Clinical studies have shown that Arixtra is effective in preventing deep vein thrombosis and pulmonary embolism in patients undergoing orthopedic surgery. Research by Turpie AG et al. demonstrated the superiority of fondaparinux over enoxaparin in preventing venous thromboembolism.
In a study by Eriksson BI et al., Arixtra exhibited a significant reduction in the incidence of venous thromboembolism compared to placebo, further supporting its efficacy and clinical utility.