Description
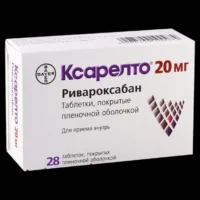
Xarelto (rivaroxaban) Coated Tablets 15 mg. №42
Composition
Each coated tablet contains 15 mg of rivaroxaban.
Mechanism of Action
Rivaroxaban is a direct Factor Xa inhibitor that selectively blocks the active site of Factor Xa, interrupting the intrinsic and extrinsic pathways of the blood coagulation cascade. This action prevents thrombin formation, reducing the risk of blood clot formation.
Pharmacological Properties
Rivaroxaban acts as an anticoagulant by inhibiting Factor Xa, a crucial enzyme in the coagulation cascade. It exhibits predictable pharmacokinetics and pharmacodynamics with once-daily dosing.
Indications for Use
Xarelto 15 mg tablets are indicated for the prevention of venous thromboembolism in adult patients undergoing elective hip or knee replacement surgery.
Contraindications
Xarelto should not be used in individuals with active pathological bleeding or lesions at risk of bleeding. It is contraindicated in patients with hepatic impairment or those receiving concomitant treatment with strong inhibitors of both CYP3A4 and P-glycoprotein.
Side Effects
Common side effects of Xarelto include bleeding, bruising, anemia, and dizziness. Severe side effects may include spinal/epidural hematoma, which can lead to long-term or permanent paralysis.
Usage Instructions
The recommended dosage is one 15 mg tablet taken orally once daily with the evening meal. The tablet should be swallowed whole with water and should not be crushed, chewed, or broken.
Benefits Compared to Analogues
Xarelto has demonstrated non-inferiority to traditional anticoagulants like enoxaparin in preventing venous thromboembolism post-orthopedic surgery, with a lower risk of major bleeding events.
Suitable Patient Groups
Xarelto can be used in adult patients undergoing elective hip or knee replacement surgery. It should be used with caution in the elderly, as well as in patients with renal impairment.
Storage and Shelf Life
Xarelto 15 mg tablets should be stored at room temperature, away from moisture and heat. The shelf life of the product should be checked before use, and expired tablets should be discarded appropriately.
Packaging Description
The packaging contains 42 coated tablets of Xarelto 15 mg each. The tablets are typically blister-packed to ensure individual tablet integrity.
Clinical Evidence and Proven Effectiveness
Rivaroxaban has been extensively studied in clinical trials, such as the RECORD program, demonstrating its efficacy and safety in preventing venous thromboembolism post-orthopedic surgery. These studies have shown that rivaroxaban is an effective alternative to traditional anticoagulants, with a favorable safety profile.