Description
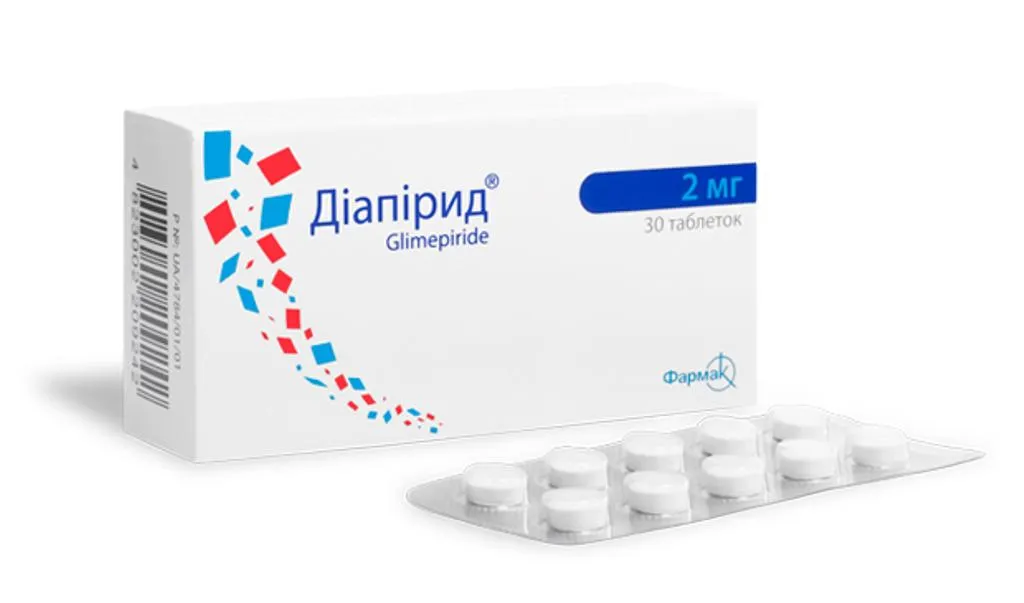
Diapirid (Glimepiride) Tablets 2 mg. №30
Ingredients
Active ingredient: Glimepiride 2 mg per tablet.
Mechanism of Action
Glimepiride, a sulfonylurea, acts by stimulating insulin release from pancreatic beta cells. This mechanism leads to increased insulin levels, which in turn lowers blood glucose levels by enhancing cellular glucose uptake and utilization.
Pharmacological Properties
Glimepiride exerts its antidiabetic effects by binding to specific receptors on pancreatic beta cells, resulting in the closure of ATP-sensitive potassium channels and subsequent depolarization of the cell membrane. This cascade of events triggers calcium influx, leading to insulin secretion.
Indications for Use
Indications: Diapirid tablets are indicated for the management of type 2 diabetes mellitus in cases where dietary modifications, physical activity, and weight management alone are insufficient to control blood sugar levels effectively.
Contraindications
Contraindications: Diapirid tablets should not be administered to individuals with a known hypersensitivity to glimepiride or other sulfonylureas. Patients with a history of sulfonylurea-induced hypoglycemia should also avoid its use.
Side Effects
Common side effects associated with Diapirid tablets include hypoglycemia, weight gain, gastrointestinal disturbances (such as nausea and diarrhea), and hypersensitivity reactions. Severe adverse effects may include hemolytic anemia, hepatic dysfunction, and skin reactions like Stevens-Johnson syndrome.
Usage Instructions
Dosage: The typical initial dose of Diapirid is 1-2 mg taken once daily, preferably with breakfast or the first substantial meal of the day. Dosage adjustments should be made based on individual blood glucose levels and response to treatment.
Benefits Compared to Analogues
Compared to other sulfonylureas, glimepiride offers a more potent and prolonged glucose-lowering effect with a lower risk of hypoglycemia. Its once-daily dosing regimen enhances patient compliance and simplifies treatment regimens, contributing to better glycemic control.
Suitable Patient Groups
Diapirid tablets are suitable for adult patients with type 2 diabetes mellitus who require pharmacological intervention to manage their condition. Caution should be exercised when prescribing to elderly individuals or those with hepatic or renal impairment, as dosage adjustments may be necessary.
Storage and Shelf Life
Store Diapirid tablets in a cool, dry place away from direct sunlight and moisture. Ensure that the packaging is tightly closed to protect the tablets from air and humidity. Check the expiration date on the packaging and do not use the product beyond the stated shelf life.
Packaging Description
Each package of Diapirid contains 30 tablets of 2 mg strength. The tablets are typically blister-packed to maintain their stability and protect them from environmental factors. The packaging should include essential information such as the drug name, dosage strength, batch number, and expiration date.
Scientific Evidence
Glimepiride has been extensively studied for its efficacy in managing type 2 diabetes mellitus. Clinical trials have demonstrated that glimepiride, as monotherapy or in combination with other antidiabetic agents, significantly reduces HbA1c levels and improves overall glycemic control in diabetic patients.
Research published in the Journal of Diabetes Investigation highlighted the positive impact of glimepiride on glucose metabolism and insulin sensitivity, emphasizing its role in enhancing long-term glycemic outcomes in individuals with type 2 diabetes.
Additional Information
Regular monitoring of blood glucose levels is crucial while using Diapirid tablets to ensure optimal diabetes management. In the event of hypoglycemia, immediate consumption of a glucose source is recommended, followed by medical consultation.
Prior to initiating Diapirid therapy, patients should disclose all concurrent medications, supplements, and medical conditions to mitigate the risk of drug interactions or adverse effects. Close medical supervision and regular follow-ups are essential to monitor treatment response and adjust the dosage as needed.