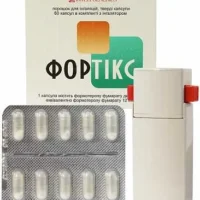
Description
Flutixon (Fluticasonum) Hard Capsules 125 mcg. №60 Powder for Inhalation
Composition
Each hard capsule contains 125 mcg of fluticasonum.
Mechanism of Action
Flutixon, with fluticasonum as the active ingredient, is a corticosteroid with potent anti-inflammatory properties. It functions by inhibiting various cell types and mediators involved in the asthmatic response, resulting in decreased airway inflammation and enhanced lung function.
Pharmacological Properties
Flutixon exerts its pharmacological effects by reducing inflammation in the airways, thereby preventing asthma symptoms and exacerbations. It helps in maintaining optimal lung function and improving overall respiratory health.
Indications for Use
Flutixon is indicated for the maintenance treatment of asthma as prophylactic therapy to prevent asthma attacks and manage chronic symptoms effectively.
Contraindications
Avoid using Flutixon if there is a known hypersensitivity to fluticasonum or any of the excipients present in the formulation. It is essential to consider this contraindication to prevent adverse reactions.
Side Effects
Common side effects of Flutixon may include throat irritation, coughing, and fungal infections in the mouth. In rare cases, it may lead to systemic effects such as adrenal suppression or allergic reactions. Patients should be monitored for any adverse events.
Usage Instructions
The recommended dosage of Flutixon is one capsule for oral inhalation once daily. Patients should carefully follow the instructions provided in the package leaflet for correct administration and optimal therapeutic outcomes.
Benefits Compared to Analogues
Compared to other asthma medications, Flutixon offers superior anti-inflammatory effects and better control of asthma symptoms. Its once-daily dosing regimen enhances patient compliance and ensures consistent asthma management.
Suitable Patient Groups
Flutixon is suitable for both pediatric and adult patients with asthma. It is particularly beneficial for elderly individuals who may require a convenient and effective treatment option for long-term asthma control.
Storage and Shelf Life
Store Flutixon in a cool, dry place away from direct sunlight. Ensure proper sealing of the packaging to maintain product integrity. Check the expiration date before use and discard any expired capsules.
Packaging Description
Flutixon is available in packs of 60 hard capsules for inhalation. The packaging is designed to protect the capsules from moisture and contamination, ensuring their stability and efficacy until the expiration date.
Scientific Evidence
Studies have demonstrated the effectiveness of Flutixon in reducing asthma exacerbations, improving lung function, and enhancing the quality of life in asthma patients. Regular use of Flutixon as prescribed has shown significant benefits in controlling asthma symptoms and minimizing disease progression.
Additional Information
It is important to emphasize that Flutixon is not intended for the relief of acute bronchospasm. Patients should be educated to have a rescue inhaler available for immediate symptom relief during acute asthma attacks. Regular monitoring of lung function and asthma symptoms is essential for optimizing treatment outcomes and ensuring adequate asthma control.