Description
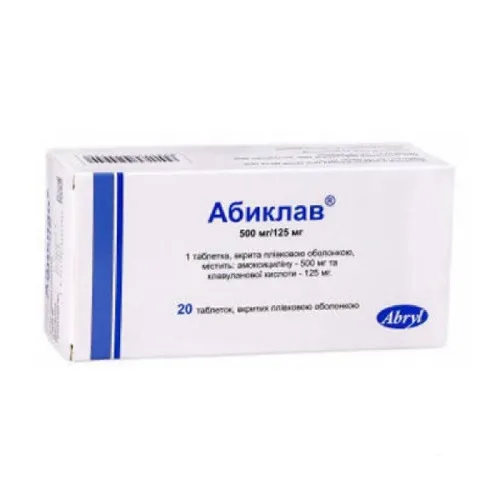
Abiklav (Amoxicillin) Coated Tablets 500 mg/125 mg. №20
Ingredients
- Each tablet contains:
- Amoxicillin (500 mg)
- Clavulanic acid (125 mg)
Dosage
The usual dose is one tablet every 8 hours. Dosage may vary based on the severity of the infection.
Indications
Abiklav tablets are indicated for the treatment of infections caused by susceptible bacteria. These include respiratory tract infections, urinary tract infections, skin and soft tissue infections, and more.
Contraindications
Do not use Abiklav if you are allergic to penicillin or cephalosporin antibiotics. Consult your doctor if you have a history of liver problems.
Directions
Swallow the tablet whole with a full glass of water. Do not crush or chew the tablet. Take Abiklav as directed by your healthcare provider.
Scientific Evidence
Amoxicillin-clavulanate combination has shown superior efficacy in treating various infections. Studies have demonstrated the synergistic effect of amoxicillin and clavulanic acid, which extends the spectrum of activity against resistant bacteria.
Additional Information
- Abiklav is generally well-tolerated, but common side effects may include diarrhea, nausea, and skin rash.
- It is important to complete the full course of treatment as prescribed by your doctor to ensure the infection is fully eradicated.
- Before using Abiklav, inform your healthcare provider about any existing medical conditions or medications you are taking. This will help prevent potential drug interactions and ensure the safety and effectiveness of the treatment.