Description
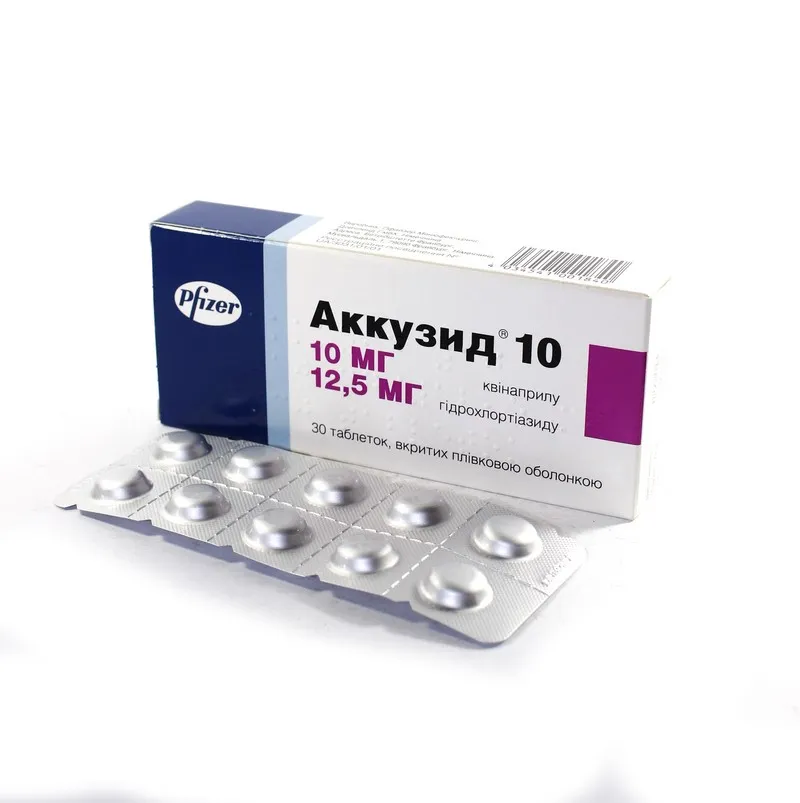
Akkuzid-10 (Quinaprilum) Coated Tablets 10 mg/12.5 mg. №30
Ingredients
- Active ingredient: Quinaprilum
- Other ingredients: lactose monohydrate, crospovidone, microcrystalline cellulose, colloidal anhydrous silica, magnesium stearate, hypromellose, macrogol 400, titanium dioxide (E171), iron oxide red (E172)
Dosage
Recommended dosage: 1 tablet daily, or as directed by a healthcare professional. Swallow the tablet whole with a glass of water.
Indications
Akkuzid-10 (Quinaprilum) coated tablets are indicated for the treatment of hypertension and congestive heart failure.
Contraindications
Do not use Akkuzid-10 tablets if:
- You are allergic to quinaprilum or any other ingredients in the product.
- You have a history of angioedema related to previous ACE inhibitor therapy.
- You are pregnant or breastfeeding.
Directions
Follow these directions for optimal results:
- Take the tablet at the same time each day.
- Avoid consuming potassium supplements or salt substitutes without consulting a healthcare provider.
- Monitor blood pressure regularly.
Scientific Evidence
Quinaprilum is an ACE inhibitor that works by blocking the conversion of angiotensin I to angiotensin II, a potent vasoconstrictor. This action leads to vasodilation and decreased blood pressure, making it effective in the management of hypertension and heart failure.
Clinical trials have demonstrated the efficacy of quinaprilum in reducing blood pressure and improving cardiac function in patients with heart failure. Research published in the Journal of the American College of Cardiology showed a significant reduction in mortality and hospitalizations in patients treated with quinapril compared to placebo.
Additional Information
It is essential to inform your healthcare provider about any existing medical conditions or medications before starting Akkuzid-10 therapy. This medication may interact with certain drugs, including diuretics, leading to adverse effects.
Always adhere to the prescribed dosage and follow-up appointments to monitor your response to Akkuzid-10. In case of any unusual symptoms or side effects, seek medical attention promptly.