Description
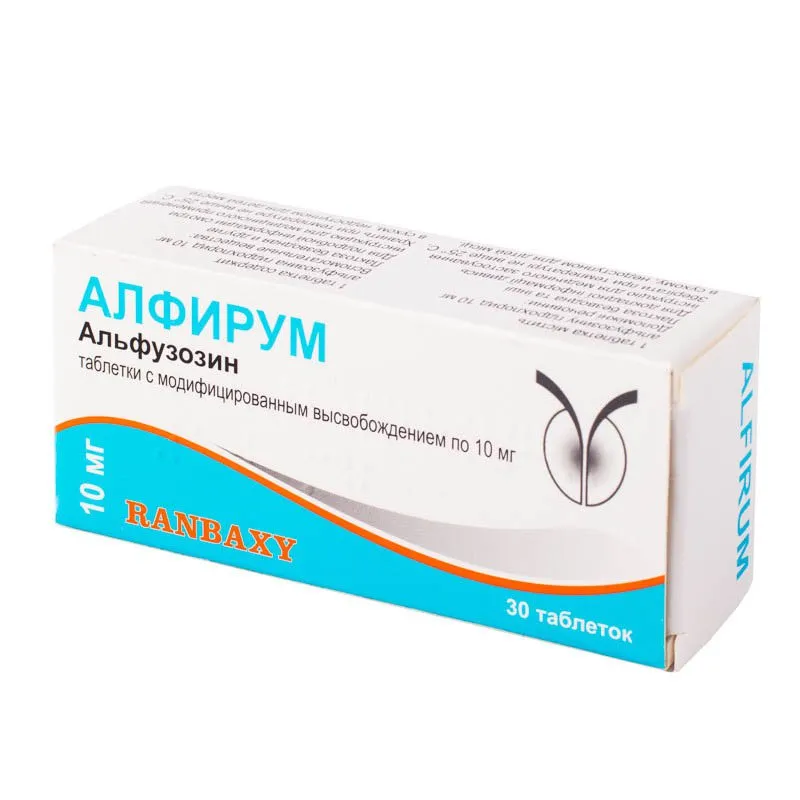
Alfirum (alfuzosin) Tablets with Modified Release 10 mg. №30
Ingredients
Active ingredient: Alfuzosin hydrochloride 10 mg.
Other ingredients: lactose monohydrate, hypromellose, colloidal anhydrous silica, magnesium stearate, macrogol, talc, titanium dioxide (E171), iron oxide yellow (E172).
Mechanism of Action
Alfuzosin, the active ingredient in Alfirum tablets, is a selective antagonist of post-synaptic alpha1-adrenoreceptors in the lower urinary tract. By blocking these receptors, alfuzosin relaxes smooth muscle in the prostate and bladder neck, leading to improved urine flow and reduced symptoms associated with benign prostatic hyperplasia (BPH).
Pharmacological Properties
Alfuzosin exerts its pharmacological effects by selectively blocking alpha1-adrenoreceptors in the prostate and bladder neck, resulting in smooth muscle relaxation and improved urinary symptoms in patients with BPH.
Indications for Use
Alfirum (alfuzosin) tablets are indicated for the treatment of benign prostatic hyperplasia (BPH) to alleviate symptoms such as difficulty urinating, weak stream, and frequent urination.
Contraindications
Do not use Alfirum (alfuzosin) tablets if you have severe liver impairment, hypotension, or a history of orthostatic reactions. Avoid concomitant use with potent CYP3A4 inhibitors as it may lead to adverse reactions.
Side Effects
Common side effects of Alfirum (alfuzosin) tablets may include dizziness, headache, and fatigue. If you experience severe dizziness or fainting, seek medical attention promptly.
Usage Instructions
Recommended dosage: Take 1 tablet daily, swallowed whole with a glass of water after the same meal each day. Do not crush or chew the tablet.
Benefits Compared to Analogues
Alfuzosin has demonstrated similar efficacy to other alpha-blockers in relieving BPH symptoms but with a lower risk of cardiovascular side effects. This makes Alfirum tablets a preferred choice for patients requiring long-term treatment for benign prostatic hyperplasia.
Suitable Patient Groups
Alfirum (alfuzosin) tablets with modified release 10 mg should be used with caution in patients with moderate hepatic impairment and in the elderly population. Consult a healthcare provider for personalized recommendations.
Storage and Shelf Life
Store Alfirum tablets in a cool, dry place away from direct sunlight. Check the expiration date on the packaging and do not use the product beyond the stated shelf life.
Packaging Description
Each package of Alfirum (alfuzosin) tablets contains 30 tablets with a modified release formulation to ensure consistent dosing and efficacy.
Clinical Evidence and Proven Effectiveness
Alfuzosin has been extensively studied in clinical trials, demonstrating its efficacy and safety in managing BPH symptoms. Research published in the Journal of Urology has highlighted the positive outcomes of alfuzosin therapy in improving urinary flow rates and quality of life in patients with benign prostatic hyperplasia.