Description
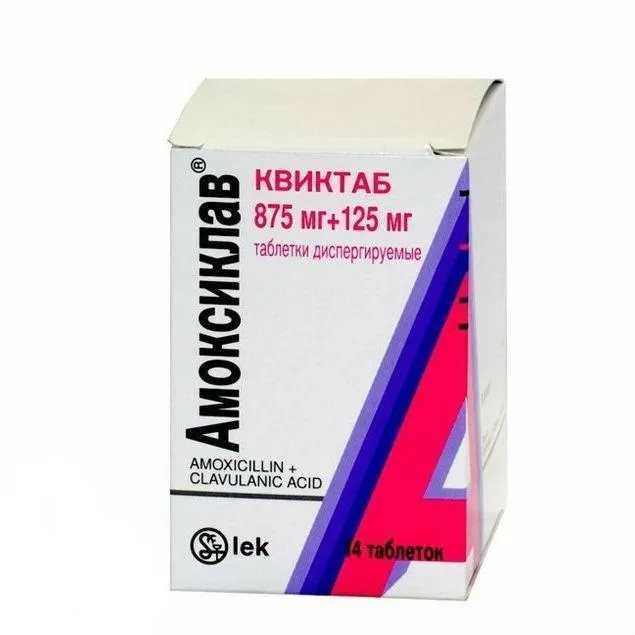
Amoxiclav (Amoxicillin) Quicktab Tablets 875 mg/125 mg. №14
Composition:
Each tablet contains 875 mg of amoxicillin and 125 mg of clavulanic acid.
Mechanism of Action:
Amoxiclav Quicktab exerts its pharmacological effects by inhibiting bacterial cell wall synthesis through the binding of amoxicillin to penicillin-binding proteins. The clavulanic acid component enhances the activity of amoxicillin by inhibiting beta-lactamase enzymes, thereby extending the spectrum of antibacterial activity.
Pharmacological Properties:
Amoxiclav Quicktab demonstrates bactericidal activity against a wide range of susceptible bacteria. The combination of amoxicillin and clavulanic acid provides synergistic effects, making it effective against beta-lactamase-producing strains.
Indications for Use:
Amoxiclav Quicktab is indicated for the treatment of various bacterial infections, including respiratory tract infections, urinary tract infections, skin and soft tissue infections, intra-abdominal infections, and gynecological infections.
Contraindications:
Avoid Amoxiclav Quicktab if you have a history of hypersensitivity reactions to penicillins, cephalosporins, or any other component of the formulation. Use caution in patients with a history of liver dysfunction or cholestatic jaundice associated with amoxicillin-clavulanate use.
Side Effects:
Common side effects of Amoxiclav Quicktab may include gastrointestinal disturbances such as diarrhea, nausea, and vomiting. Allergic reactions like rash, itching, and angioedema can occur. Severe adverse effects such as anaphylaxis and pseudomembranous colitis are rare but serious.
Usage Instructions:
Take one tablet of Amoxiclav Quicktab every 12 hours. The dosage may be adjusted based on the severity of the infection and should be administered as directed by a healthcare professional. Swallow the tablet whole with a full glass of water, with or without food.
Benefits Compared to Analogues:
Amoxiclav Quicktab offers the advantage of dual therapy with amoxicillin and clavulanic acid, providing enhanced efficacy against beta-lactamase-producing bacteria compared to traditional amoxicillin formulations.
Suitable Patient Groups:
Amoxiclav Quicktab is suitable for use in adults, adolescents, and children for the treatment of bacterial infections. Dosage adjustments may be necessary in patients with renal impairment.
Storage Conditions and Shelf Life:
Store Amoxiclav Quicktab in a cool, dry place away from direct sunlight. Keep the tablets in their original packaging to protect from moisture. Check the expiration date on the packaging and do not use the product after the specified shelf life.
Packaging Description:
Each package of Amoxiclav Quicktab contains 14 tablets of 875 mg amoxicillin and 125 mg clavulanic acid. The tablets are blister-packed for individual dosage convenience.
Scientific Evidence:
Amoxiclav Quicktab has been extensively researched and proven effective in the treatment of various bacterial infections. Clinical studies have demonstrated the superior efficacy of amoxicillin-clavulanate combination therapy in combating resistant bacterial strains and improving clinical outcomes.
Additional Information:
Completing the full course of treatment with Amoxiclav Quicktab is crucial to prevent the development of antibiotic resistance. Inform your healthcare provider about all medications you are taking to avoid potential drug interactions. If you experience any unusual symptoms or severe side effects, seek medical attention promptly.