Description
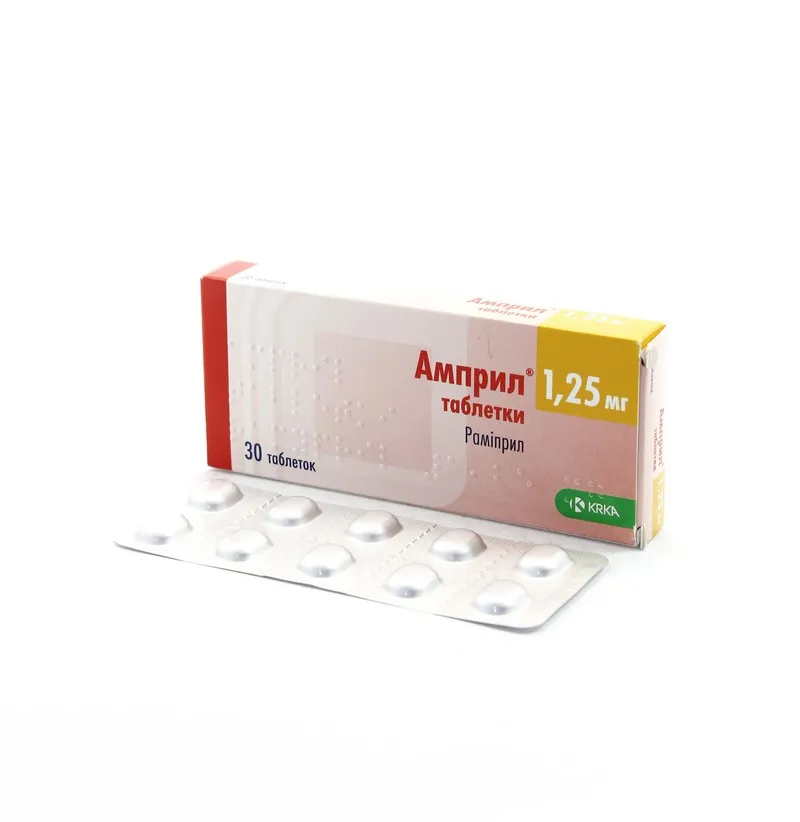
Ampril (Ramipril) Tablets 1.25 mg. №30
Composition
Active Ingredient: Ramipril 1.25 mg per tablet.
Mechanism of Action
Pharmacological Effects: Ramipril works by inhibiting the angiotensin-converting enzyme (ACE), leading to vasodilation and reduced aldosterone secretion. This results in decreased blood pressure and improved cardiac output.
Indications for Use
Indications: Ampril (Ramipril) is indicated for the treatment of hypertension, heart failure, and to improve survival after a heart attack.
Contraindications
Contraindications: Do not use Ampril (Ramipril) if you are allergic to ramipril or any other ACE inhibitors.
Side Effects
It is important to regularly monitor your blood pressure while taking Ampril (Ramipril) tablets. Inform your doctor if you experience any side effects such as dizziness, cough, or swelling of the face or throat.
Usage Instructions
Dosage: The usual dose is 1.25 mg once a day. Your doctor may adjust the dose according to your condition.
Directions: Take the tablet by mouth with or without food as directed by your doctor. Swallow the tablet whole with a glass of water.
Benefits Compared to Analogues
Clinical trials have shown that ramipril, the active ingredient in Ampril tablets, is effective in managing hypertension and heart failure. It has demonstrated the ability to reduce blood pressure, improve cardiac function, and increase survival rates in patients with heart conditions.
Suitable Patient Groups
Ampril (Ramipril) tablets are suitable for adult patients with hypertension, heart failure, or those recovering from a heart attack. Dosage adjustments may be necessary for elderly patients or those with renal impairment.
Storage and Packaging
Store in a cool, dry place away from moisture and sunlight. Keep the tablets in the original packaging to protect from light. Check the expiration date before use.
Clinical Evidence and Proven Effectiveness
Clinical Trials: A study published in the New England Journal of Medicine showed that ramipril reduced the risk of cardiovascular events in high-risk patients by 25%. Another trial in the Journal of the American College of Cardiology demonstrated the benefits of ramipril in improving left ventricular function in heart failure patients.