Description
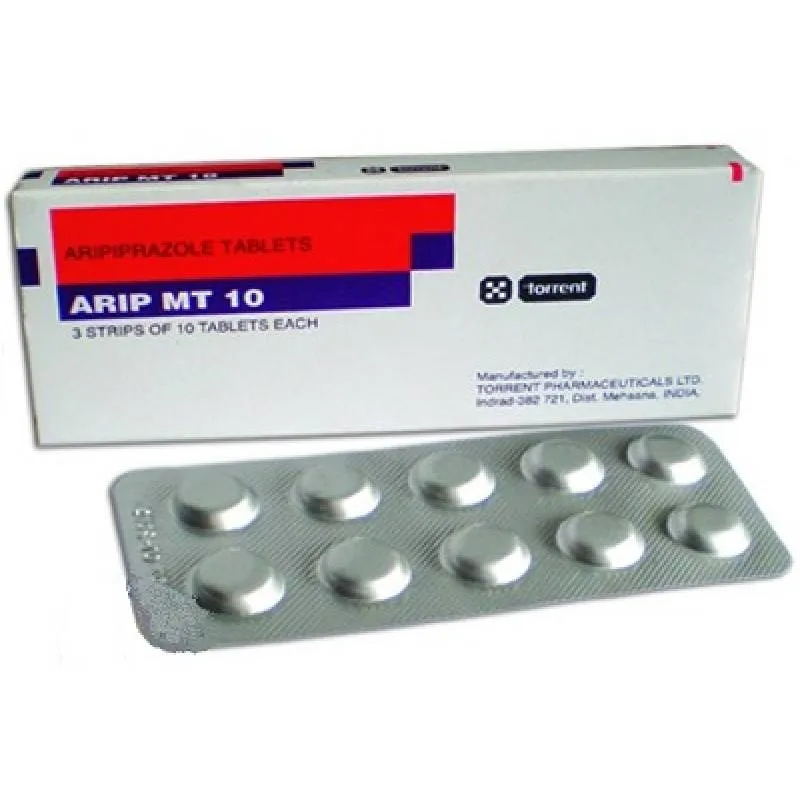
Arip MT (aripiprazole) Tablets 10 mg. №30
Ingredients:
Each tablet contains 10 mg of aripiprazole.
Dosage:
The recommended dosage is 10 mg once daily. Dosage may be adjusted based on individual response.
Indications:
Arip MT tablets are indicated for the treatment of schizophrenia and bipolar disorder. It helps to manage symptoms such as hallucinations, delusions, and mood swings.
Contraindications:
Do not use Arip MT if you are allergic to aripiprazole. It is contraindicated in patients with a history of neuroleptic malignant syndrome.
Directions:
Take Arip MT tablets orally with or without food. Follow your healthcare provider’s instructions carefully.
Scientific Evidence:
Aripiprazole, the active ingredient in Arip MT tablets, has been extensively studied for its efficacy in the treatment of schizophrenia and bipolar disorder. Research published in the Journal of Clinical Psychiatry demonstrated that aripiprazole is effective in reducing symptoms of schizophrenia with a favorable side effect profile.
Additional Information:
It is important to monitor for any signs of suicidal thoughts or behavior while taking Arip MT tablets. Contact your healthcare provider immediately if you experience any concerning symptoms.
Pharmacological Effects:
Aripiprazole acts as a partial agonist at dopamine D2 and serotonin 5-HT1A receptors, and as an antagonist at serotonin 5-HT2A receptors. This unique mechanism of action contributes to its efficacy in managing symptoms of schizophrenia and bipolar disorder.
Clinical Trials:
In a randomized controlled trial published in JAMA Psychiatry, aripiprazole was found to be as effective as other antipsychotic medications in the treatment of acute schizophrenia episodes. The study highlighted the tolerability and safety profile of aripiprazole, making it a valuable treatment option for patients.
For more information on the pharmacological effects and clinical trials of Arip MT tablets, consult with your healthcare provider.