Description
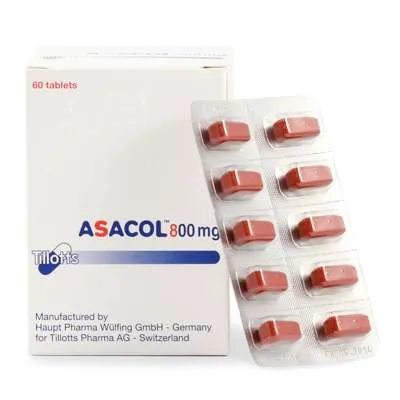
Asacol (Mesalazine) Coated Enteric Tablets 800 mg. №60
Composition
Each tablet contains 800 mg of mesalazine.
Mechanism of Action
Mesalazine, the active ingredient in Asacol tablets, acts as a locally acting anti-inflammatory agent in the colon. It inhibits the production of inflammatory mediators and exerts its therapeutic effects directly at the site of inflammation.
Pharmacological Properties
Asacol tablets containing mesalazine possess anti-inflammatory, analgesic, and antioxidant properties. Mesalazine modulates the immune response in the colon, reducing inflammation and promoting mucosal healing.
Indications for Use
Asacol tablets are indicated for the treatment of mild to moderate ulcerative colitis. They are effective in inducing and maintaining remission, reducing symptoms such as diarrhea, rectal bleeding, and abdominal pain.
Contraindications
Avoid using Asacol if you have a known hypersensitivity to mesalazine or any other components of the tablets. Patients with severe renal impairment, hepatic dysfunction, or blood dyscrasias should also avoid using this medication.
Side Effects
- Common side effects may include headache, nausea, abdominal pain, and flatulence.
- Less common side effects may include pancreatitis, liver function abnormalities, and allergic reactions.
- In rare cases, Asacol may cause worsening colitis symptoms or kidney disorders.
Usage Instructions
The typical dosage regimen involves taking 2 to 6 tablets of Asacol daily, divided into multiple doses. Swallow the tablets whole with water, without crushing or chewing them, to ensure proper delivery of the medication to the colon.
Benefits Compared to Analogues
Asacol offers the advantage of targeted drug delivery to the colon due to its enteric coating. This feature minimizes systemic absorption, reducing the risk of systemic side effects commonly associated with other oral formulations of mesalazine.
Suitable Patient Groups
Asacol tablets are suitable for use in adult patients, including the elderly population, for the management of ulcerative colitis. The dosage may need adjustment in patients with renal impairment to prevent drug accumulation.
Storage and Shelf Life
Store Asacol tablets in a cool, dry place away from moisture and heat. Check the expiration date on the packaging and do not use the tablets beyond the specified shelf life to ensure efficacy and safety.
Packaging Description
Asacol tablets are packaged in blister packs containing 60 coated enteric tablets, each with 800 mg of mesalazine. The packaging is designed to protect the tablets from environmental factors and maintain their stability.
Clinical Evidence and Proven Effectiveness
Studies have demonstrated the efficacy of mesalazine in inducing and maintaining remission in patients with ulcerative colitis. Research published in the Journal of Crohn’s and Colitis highlighted the anti-inflammatory properties of mesalazine, supporting its role in reducing colonic inflammation and improving clinical outcomes.