Description
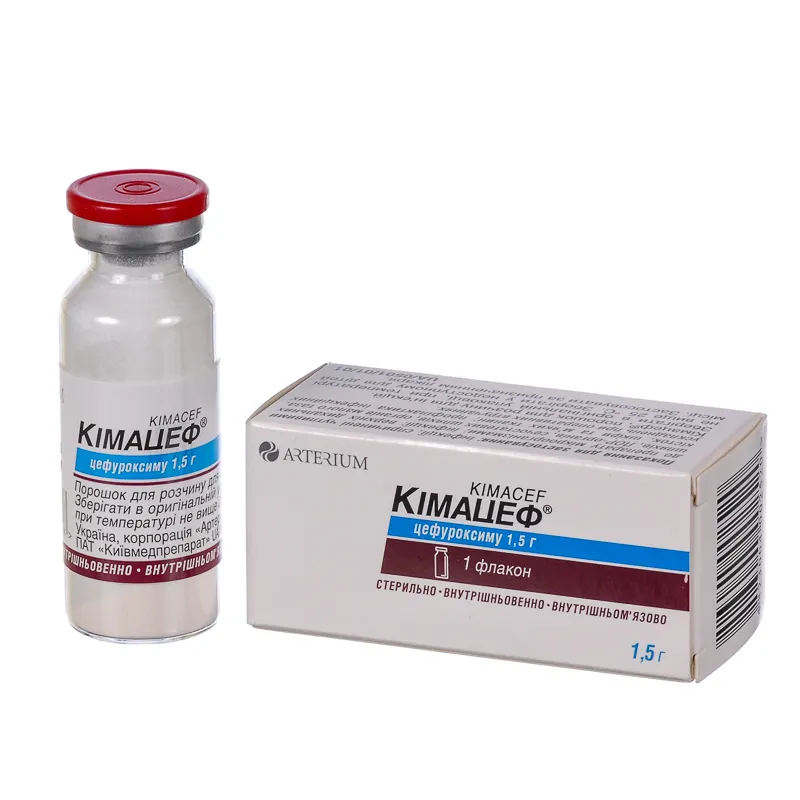
Kimacef (Cefuroxime) 1.5 g Vial for Intravenous Administration
Composition
Active Ingredient: Cefuroxime. Other ingredients may include sodium carbonate, sodium citrate, and water for injection.
Mechanism of Action
Pharmacological Properties: Cefuroxime exerts its effects by disrupting the synthesis of the bacterial cell wall, resulting in bacterial cell death. It demonstrates bactericidal activity against a broad spectrum of gram-positive and gram-negative bacteria.
Indications for Use
Indicated for: Kimacef is prescribed for the treatment of infections caused by susceptible bacteria. This includes infections of the lower respiratory tract, skin and skin structure, urinary tract, bone and joint, and septicemia.
Contraindications
Contraindications: Kimacef should not be administered to individuals with a known hypersensitivity to cefuroxime or other cephalosporins.
Side Effects
Common side effects of Kimacef may include gastrointestinal disturbances, allergic reactions, and skin rashes. Patients should seek medical advice if they experience any adverse reactions.
Usage Instructions
Administration: Kimacef is administered via intravenous infusion over a 30-minute period. The dosage and duration of therapy should be individualized based on the severity of the infection.
Benefits Compared to Analogues
Kimacef offers a broad spectrum of antibacterial activity, making it effective against a wide range of pathogens. Its proven efficacy and safety profile make it a valuable option in the management of serious infections.
Suitable Patient Groups
Kimacef can be used in various patient populations, including adults, children, and the elderly. Dosage adjustments may be necessary based on age and renal function.
Storage and Shelf Life
Kimacef should be stored as per the manufacturer’s instructions. It is essential to check the expiration date on the packaging and discard any expired vials. Proper storage conditions help maintain the product’s stability and effectiveness.
Packaging Description
Kimacef is available in single-dose vials containing 1.5 grams of cefuroxime for intravenous administration. The packaging should be inspected for any signs of damage before use.
Clinical Evidence and Proven Effectiveness
Clinical studies have demonstrated the efficacy of cefuroxime in treating various bacterial infections. Research published in reputable journals, such as the Journal of Antimicrobial Chemotherapy and the International Journal of Infectious Diseases, supports the effectiveness and safety of cefuroxime in clinical practice.