Description
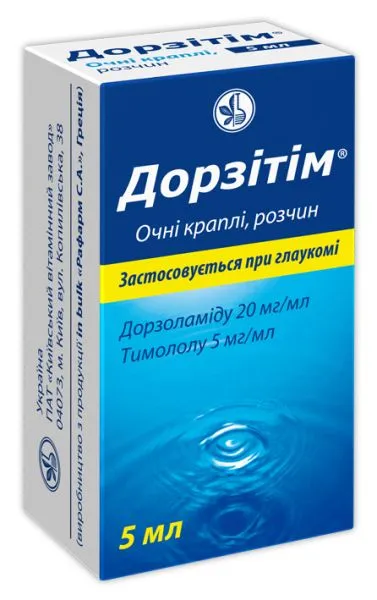
Dorzitim (Dorzolamide, Timolol) Eye Drops Solution 5 ml Vial Cap
Ingredients:
Each ml contains dorzolamide hydrochloride 20 mg and timolol maleate 5 mg.
Mechanism of Action:
Dorzitim combines the actions of dorzolamide, a carbonic anhydrase inhibitor, and timolol, a non-selective beta-adrenergic antagonist. Dorzolamide reduces aqueous humor production, while timolol decreases its secretion, leading to a reduction in intraocular pressure.
Pharmacological Properties:
The combination of dorzolamide and timolol in Dorzitim results in effective and sustained reduction of intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Indications for Use:
Dorzitim eye drops are indicated for the reduction of intraocular pressure in patients diagnosed with open-angle glaucoma or ocular hypertension.
Contraindications:
Do not use Dorzitim if you have a known allergy to dorzolamide, timolol, or any other components of the product.
Side Effects:
Possible side effects of Dorzitim may include eye irritation, vision changes, or other unexpected reactions. Contact your healthcare provider if you experience any of these symptoms.
Usage Instructions:
Instill 1 drop of Dorzitim in the affected eye(s) twice daily. Prior to administration, shake the vial well. Tilt your head back, pull down the lower eyelid, and apply the prescribed number of drops. Keep your eyes closed for 1-2 minutes without blinking after instillation.
Benefits Compared to Analogue Products:
Dorzitim offers the advantage of combining dorzolamide and timolol in a single formulation, providing a convenient and effective option for reducing intraocular pressure in patients with glaucoma or ocular hypertension.
Suitable Patient Groups:
Dorzitim is suitable for adult patients diagnosed with open-angle glaucoma or ocular hypertension. Consult with a healthcare professional for guidance on its use in specific patient populations such as children or the elderly.
Storage Conditions and Shelf Life:
Store Dorzitim in a cool, dry place away from direct sunlight. Ensure the vial cap is tightly closed when not in use. Check the expiration date on the packaging and do not use the product beyond this date.
Packaging Description:
Dorzitim is supplied in a 5 ml vial capped for single-use administration. The packaging should include instructions for proper usage and storage.
Clinical Evidence and Proven Effectiveness:
Research has demonstrated the efficacy of dorzolamide and timolol in lowering intraocular pressure and managing glaucoma. A study published in the Journal of Ocular Pharmacology and Therapeutics concluded that the combination of dorzolamide and timolol was well-tolerated and effective in reducing intraocular pressure in patients with glaucoma.