Description
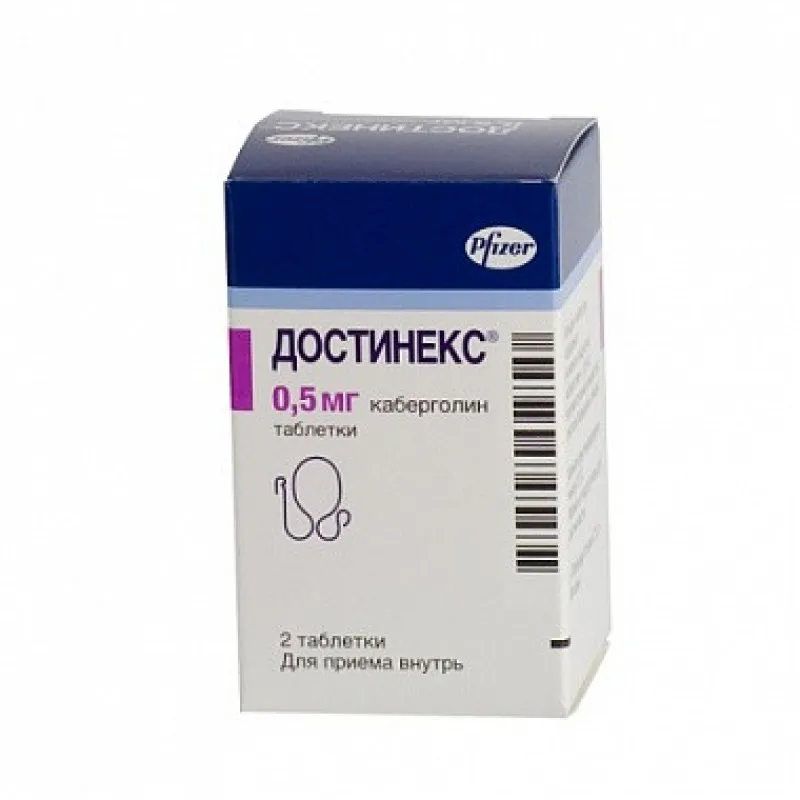
Dostinex (Cabergoline) Tablets 0.5 mg
Composition
Active ingredient: Cabergoline 0.5 mg per tablet.
Mechanism of Action
Cabergoline, the active ingredient in Dostinex tablets, is a dopamine receptor agonist. It works by stimulating dopamine receptors in the brain, specifically the D2 receptors, leading to inhibition of prolactin secretion from the pituitary gland.
Pharmacological Properties
Dostinex tablets contain cabergoline, which has a long duration of action due to its high affinity for D2 receptors. It exerts its inhibitory effect on prolactin secretion, thereby reducing serum prolactin levels.
Indications for Use
Dostinex tablets are indicated for the treatment of hyperprolactinemia, a condition characterized by elevated levels of prolactin hormone in the blood. They are also used in the management of disorders associated with hyperprolactinemia, such as amenorrhea, oligomenorrhea, galactorrhea, and infertility.
Contraindications
Do not use Dostinex if you have a known hypersensitivity to cabergoline or any other components of the product. It is essential to consult a healthcare provider before initiating treatment with Dostinex, especially if you have a history of psychiatric disorders or uncontrolled hypertension.
Side Effects
The common side effects associated with Dostinex include nausea, dizziness, constipation, fatigue, and headache. In rare cases, it may cause psychiatric symptoms such as hallucinations or compulsive behaviors. If you experience any severe side effects, seek medical attention promptly.
Usage Instructions
Take Dostinex tablets orally as prescribed by your healthcare provider. The usual recommended dose is 0.5 mg twice a week, but the dosage may vary depending on individual response and medical condition. It can be taken with or without food. Do not exceed the prescribed dose to avoid potential adverse effects.
Benefits Compared to Analogues
Dostinex, containing cabergoline as the active ingredient, offers superior efficacy in normalizing prolactin levels and improving symptoms of hyperprolactinemia compared to other dopamine agonists. Its longer half-life and potent D2 receptor binding affinity contribute to its effectiveness in managing hyperprolactinemia-related conditions.
Suitable Patient Groups
Dostinex is suitable for adult patients, including women of childbearing age and men, who require treatment for hyperprolactinemia-related disorders. It is not recommended for use in children, pregnant women, or individuals with a history of cardiac valve disorders.
Storage and Shelf Life
- Storage: Keep Dostinex tablets at room temperature, protected from light and moisture. Avoid storing them in the bathroom or kitchen where humidity levels can fluctuate.
- Shelf Life: Check the expiration date on the packaging and do not use the tablets if they have exceeded their shelf life.
Packaging Description
Dostinex tablets are typically available in blister packs containing 2 tablets each. The packaging is designed to protect the tablets from external factors that could compromise their integrity and efficacy.
Clinical Evidence and Proven Effectiveness
Dostinex (Cabergoline) tablets 0.5 mg have undergone extensive clinical studies to evaluate their efficacy in treating hyperprolactinemia. Research published in the Journal of Clinical Endocrinology & Metabolism has demonstrated that cabergoline effectively reduces prolactin levels in patients with hyperprolactinemia, leading to improvements in menstrual irregularities and infertility.