Description
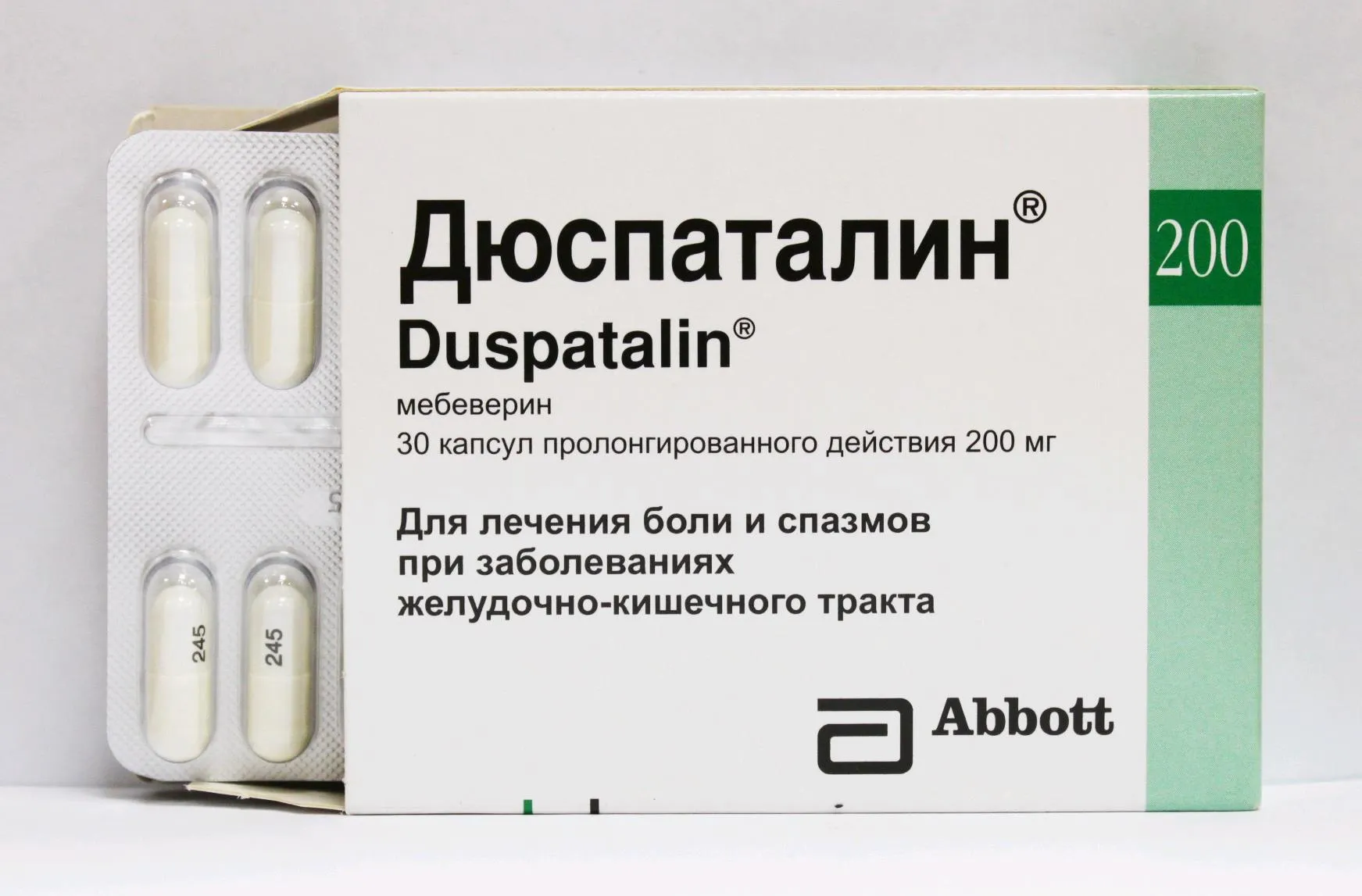
Duspatalin Coated Tablets 135 mg. №15
Ingredients:
Each coated tablet contains 135 mg of Mebeverine Hydrochloride.
Mechanism of Action:
Mebeverine, the active ingredient in Duspatalin, acts as a musculotropic antispasmodic agent by directly affecting the smooth muscle in the gastrointestinal tract.
Pharmacological Properties:
Mebeverine exerts its therapeutic effects by inhibiting excessive contractions of the gut muscles, thereby reducing spasms and associated symptoms.
Indications for Use:
Duspatalin tablets are specifically indicated for the treatment of irritable bowel syndrome (IBS) and related gastrointestinal disorders characterized by abdominal pain, bloating, and altered bowel habits.
Contraindications:
Avoid using Duspatalin if you have a known allergy to Mebeverine Hydrochloride or any other components present in the tablets. Consult a healthcare professional before starting treatment if uncertain.
Side Effects:
Common side effects of Duspatalin may include mild gastrointestinal disturbances such as nausea, constipation, or diarrhea. In rare cases, allergic reactions like rash or itching may occur. Discontinue use and seek medical advice if any adverse reactions manifest.
Usage Instructions:
The typical dosage for adults is one 135 mg tablet taken orally three times daily, ideally before meals. Swallow the tablets whole with a sufficient amount of water. Avoid breaking or crushing the tablets.
Benefits Compared to Analogues:
When compared to other antispasmodic agents, Duspatalin has demonstrated similar efficacy in managing IBS symptoms while exhibiting a more favorable safety profile with reduced risk of adverse effects. This makes Duspatalin a preferred choice for healthcare providers in treating IBS.
Suitable Patient Groups:
Duspatalin is generally well-tolerated across different age groups, including children, adults, and the elderly. However, individual dosing adjustments may be necessary based on age, medical history, and overall health status.
Storage and Shelf Life:
Store Duspatalin tablets in a cool, dry place away from direct sunlight and moisture. Ensure the packaging is tightly closed. Check the expiration date on the blister pack and do not use the tablets beyond the stated shelf life.
Packaging Description:
Duspatalin is available in blister packs containing 15 coated tablets, each tablet marked with the dosage strength of 135 mg. The packaging is designed to maintain the stability and integrity of the tablets until consumption.
Clinical Evidence and Proven Effectiveness:
Studies have shown that Mebeverine, the key component of Duspatalin, effectively reduces the frequency and severity of abdominal pain, bloating, and discomfort associated with IBS. Clinical trials have demonstrated the significant symptom relief and improved quality of life experienced by patients using Duspatalin for IBS management.