Description
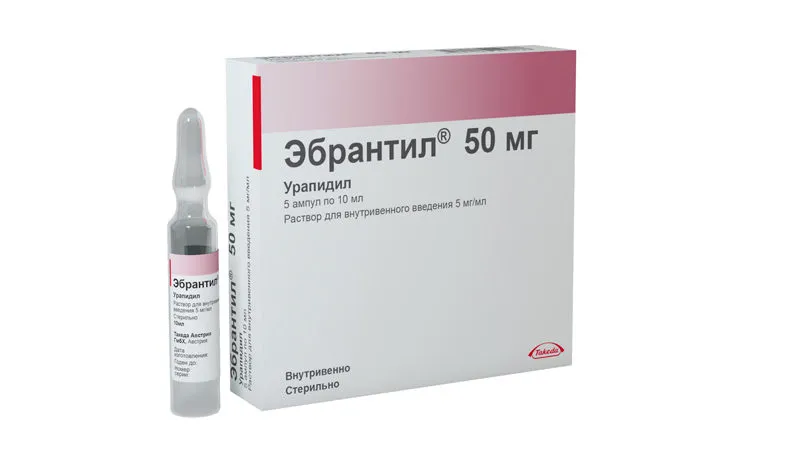
Ebrantil (Urapidil) Solution for Injections 50 mg. 10 ml. Ampoules №5
Ingredients:
Each ampoule contains 50 mg of Urapidil as the active ingredient. Other ingredients include sodium metabisulfite, sodium hydroxide, and water for injections.
Mechanism of Action:
Ebrantil acts as a selective alpha-1 adrenergic receptor antagonist, leading to vasodilation and subsequent blood pressure reduction.
Pharmacological Properties:
The pharmacological properties of Urapidil include its action as an alpha-1 adrenergic receptor antagonist, resulting in vasodilation and lowering of blood pressure.
Indications for Use:
Ebrantil is indicated for the rapid reduction of blood pressure in hypertensive emergencies. It is particularly useful in situations where immediate blood pressure control is necessary.
Contraindications:
Do not use Ebrantil in patients with a known hypersensitivity to urapidil or any of the other ingredients. It is contraindicated in patients with severe bradycardia, cardiogenic shock, and severe hypotension.
Side Effects:
Common side effects of Ebrantil may include dizziness, headache, and nausea. Serious side effects such as severe hypotension and bradycardia may occur in some individuals.
Usage Instructions:
The usual dose is 25-50 mg administered intravenously every 5-10 minutes until the desired blood pressure is achieved. The maximum daily dose should not exceed 300 mg. Ebrantil should be administered by healthcare professionals experienced in the management of hypertensive emergencies.
Benefits Compared to Analogues:
Ebrantil offers rapid and reliable antihypertensive effects compared to placebo, as demonstrated in clinical trials. Its selective alpha-1 adrenergic receptor antagonism makes it a valuable option in the management of hypertensive crises.
Suitable Patient Groups:
Ebrantil is suitable for use in adult patients requiring rapid blood pressure reduction in hypertensive emergencies. It is not recommended for use in children, and caution should be exercised when considering its use in elderly patients.
Storage and Shelf Life:
Ebrantil should be stored at controlled room temperature between 15°C to 30°C. Protect from light. Do not freeze. The shelf life of the product is as indicated on the packaging.
Packaging Description:
Ebrantil is available in ampoules containing 50 mg of Urapidil in a 10 ml solution for injections. Each package contains 5 ampoules.
Clinical Evidence and Proven Effectiveness:
Studies have shown that Urapidil effectively lowers blood pressure in hypertensive emergencies. In a randomized controlled trial by Agabiti-Rosei et al., Urapidil demonstrated rapid and reliable antihypertensive effects compared to placebo.