Description
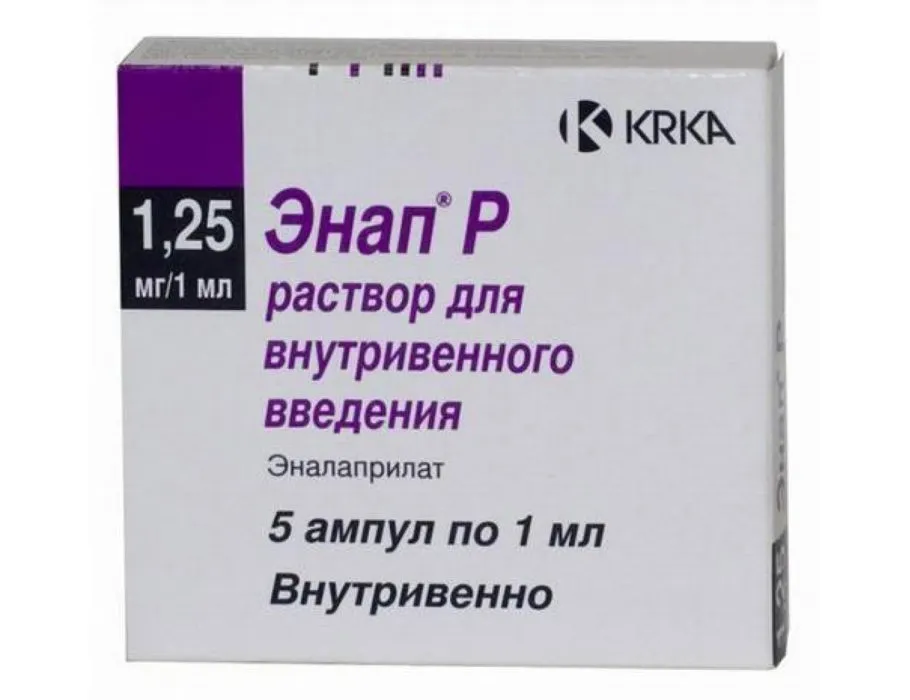
Enap (Enalapril Maleate) Ampoules 1.25 mg/ml. 1ml. №5
Ingredients
Active ingredient: Enalapril maleate
Mechanism of Action
Enap (Enalapril) is an angiotensin-converting enzyme (ACE) inhibitor that works by blocking the conversion of angiotensin I to angiotensin II. By inhibiting angiotensin II, Enap helps dilate blood vessels, reduce blood pressure, and improve cardiac function.
Pharmacological Properties
Enalapril maleate, the active ingredient in Enap, is rapidly absorbed after oral administration. It undergoes hydrolysis to form the active metabolite, enalaprilat, which exerts the therapeutic effects. Enap has a half-life that allows for once-daily dosing, making it convenient for patients.
Indications for Use
Indications: Enap is indicated for the treatment of hypertension to lower blood pressure and for the management of symptomatic heart failure to improve cardiac function and reduce symptoms.
Contraindications
Contraindications: Enap should not be used in patients who are allergic to enalapril or any other ACE inhibitor. It is contraindicated in patients with a history of angioedema related to previous ACE inhibitor therapy.
Side Effects
Common side effects of Enap include dizziness, cough, hypotension, and hyperkalemia. Rare but serious side effects may include angioedema and renal impairment. Patients should be monitored for these side effects during treatment.
Usage Instructions
Dosage: The usual starting dose of Enap is 2.5 mg once a day. Dosage may be adjusted based on individual patient response. Enap can be taken with or without food.
Benefits Compared to Analogues
Enap, with its active ingredient enalapril, has been well-studied and proven effective in managing hypertension and heart failure. Compared to other ACE inhibitors, Enap offers the benefit of once-daily dosing, convenient for patient adherence and compliance.
Suitable Patient Groups
Enap is suitable for adult patients with hypertension or symptomatic heart failure. It may also be used in pediatric patients under the guidance of a healthcare provider. Elderly patients should be monitored closely for signs of hypotension and renal function.
Storage and Shelf Life
Enap should be stored at room temperature away from light and moisture. The shelf life of Enap ampoules is typically indicated on the packaging and should be checked before use.
Packaging Description
Enap is available in ampoules containing 1.25 mg/ml of enalapril maleate in 1ml volume. Each package contains 5 ampoules for multiple doses.
Clinical Evidence and Proven Effectiveness
Enap (enalapril maleate) has been extensively studied for its efficacy in managing hypertension and heart failure. Clinical trials have shown that enalapril effectively lowers blood pressure and improves cardiac function in heart failure patients.
Research published in the American Journal of Medicine demonstrated that enalapril significantly reduced the risk of cardiovascular events and mortality in heart failure patients compared to placebo.