Description
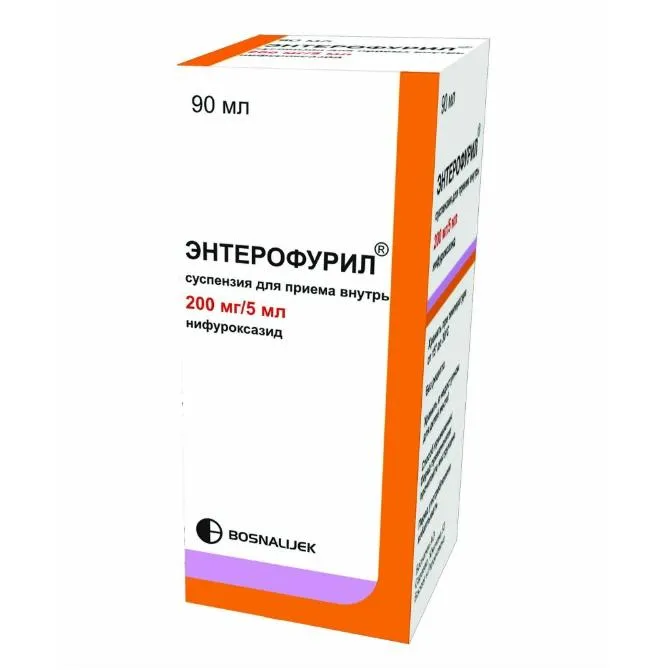
Enterofuril (Nifuroxazide) Suspension 200 mg/5 ml. 90 ml. №1
Composition
Active ingredient: Nifuroxazide. Other ingredients: sucrose, sodium benzoate, povidone, banana flavor, purified water.
Mechanism of Action
Nifuroxazide exerts its pharmacological action by selectively targeting bacterial pathogens in the gut, without disrupting the normal flora. This targeted approach helps in resolving diarrhea while preserving the beneficial bacteria in the intestines.
Pharmacological Properties
Nifuroxazide acts by inhibiting bacterial enzymes, thereby reducing the production of toxins that cause diarrhea.
Indications for Use
Enterofuril suspension is indicated for the treatment of acute infectious diarrhea of bacterial origin in adults and children.
Contraindications
Do not use Enterofuril suspension if you are allergic to nifuroxazide or any other ingredients in the product. Consult a doctor before use if pregnant or breastfeeding.
Side Effects
Enterofuril suspension is well-tolerated with minimal side effects, making it a preferred choice for the treatment of bacterial diarrhea.
Usage Instructions
Shake the bottle before use. Measure the prescribed dose using the provided measuring cup. Administer the suspension orally. Do not dilute with other liquids.
Benefits Compared to Analogues
Enterofuril suspension has demonstrated effectiveness in reducing the duration and severity of acute infectious diarrhea compared to placebo. Its rapid onset of action and minimal side effects make it a valuable option for both adults and children.
Suitable Patient Groups
Enterofuril suspension is suitable for adults and children over 6 years old. Consult a healthcare professional for appropriate dosing in children under 6.
Storage and Shelf Life
Store Enterofuril suspension in a cool, dry place away from direct sunlight. Check the expiration date on the packaging and do not use after the specified date.
Packaging Description
Enterofuril suspension is available in a 90 ml bottle with a measuring cup for accurate dosing.
Clinical Evidence and Proven Effectiveness
Nifuroxazide, the active ingredient in Enterofuril, has demonstrated efficacy in treating acute infectious diarrhea by targeting pathogenic bacteria in the gastrointestinal tract. Studies have shown that nifuroxazide acts by inhibiting bacterial enzymes, thereby reducing the production of toxins that cause diarrhea.