Description
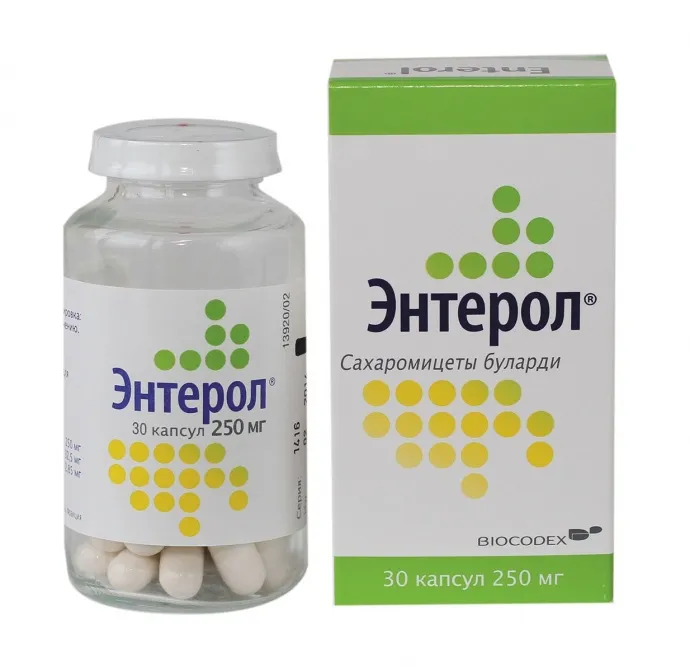
Enterol (Saccharomyces Boulardii) Capsules 250mg – Pack of 30
Composition
Each capsule contains 250mg of Saccharomyces Boulardii.
Mechanism of Action
Saccharomyces Boulardii functions as a probiotic yeast that aids in restoring the natural balance of gut microbiota. It is particularly beneficial in cases where the gut flora is disrupted, such as after antibiotic therapy or infections.
Pharmacological Properties
Saccharomyces Boulardii exhibits probiotic properties that help in maintaining a healthy gut environment. It supports gastrointestinal health by regulating the microbial balance in the intestines.
Indications for Use
Enterol capsules are recommended for the prevention and treatment of diarrhea associated with antibiotic therapy, Clostridium difficile infection, and traveler’s diarrhea.
Contraindications
Avoid using Enterol if you are immunocompromised or have a central venous catheter in place.
Side Effects
Common side effects may include mild gastrointestinal disturbances such as bloating or flatulence. Discontinue use if you experience any severe adverse reactions.
Usage Instructions
Take one capsule daily with a meal or as advised by a healthcare professional.
Benefits Compared to Analogues
Enterol offers a convenient method to supplement with Saccharomyces Boulardii, known for its efficacy in supporting gut health. Its high-quality manufacturing process ensures potency and purity.
Suitable Patient Groups
Enterol is suitable for adults, children, and the elderly. However, consult a healthcare provider before administering to individuals with specific health conditions or pregnant and breastfeeding women.
Storage Conditions and Shelf Life
Store Enterol in a cool, dry place away from direct sunlight. Ensure it is out of the reach of children. Check the expiration date on the packaging and do not use after the specified date.
Packaging Description
Enterol is available in a pack containing 30 capsules, each with 250mg of Saccharomyces Boulardii.
Clinical Evidence and Proven Effectiveness
Saccharomyces Boulardii has been extensively studied for its positive impact on gastrointestinal health. Clinical trials have shown its effectiveness in preventing and treating various forms of diarrhea. For instance, a study in the American Journal of Gastroenterology reported a significant reduction in the risk of antibiotic-associated diarrhea with Saccharomyces Boulardii supplementation.