Description
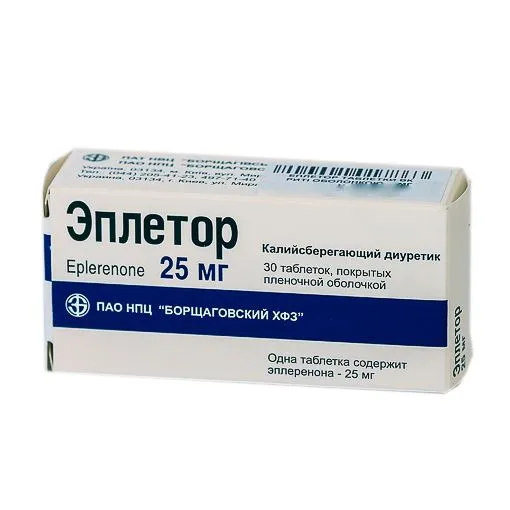
Epletor (Eplerenone) Coated Tablets 25 mg. №30
Ingredients:
Each coated tablet contains 25 mg of eplerenone as the active ingredient.
Dosage:
The usual recommended dose is one 25 mg tablet taken orally once daily.
Indications:
Epletor (eplerenone) is indicated for the treatment of hypertension and heart failure. It is often used in combination with other medications to improve outcomes in patients with heart conditions.
Contraindications:
Do not use Epletor if you are allergic to eplerenone or if you have severe kidney problems. It is important to consult with a healthcare provider before starting this medication.
Directions:
Take Epletor exactly as prescribed by your doctor. Do not adjust the dosage without medical advice. It can be taken with or without food, but consistency is key for optimal results.
Scientific Evidence:
Studies have shown that eplerenone, the active ingredient in Epletor, is effective in reducing blood pressure and improving cardiac function in patients with heart failure. Research published in the Journal of the American College of Cardiology demonstrated the benefits of eplerenone in reducing cardiovascular mortality and hospitalizations in heart failure patients.
Additional Information:
It is important to monitor potassium levels regularly while taking Epletor, as it can cause hyperkalemia (high potassium levels). Inform your healthcare provider of any side effects or concerns while on this medication.
Eplerenone works by blocking the effects of aldosterone, a hormone that can contribute to heart and kidney problems. By inhibiting aldosterone, Epletor helps to reduce sodium and water retention, leading to lower blood pressure and improved heart function.
Clinical trials have shown that Epletor is well-tolerated and effective in managing hypertension and heart failure. Compared to similar drugs, eplerenone has been associated with a lower risk of certain side effects, making it a favorable choice for many patients.