Description
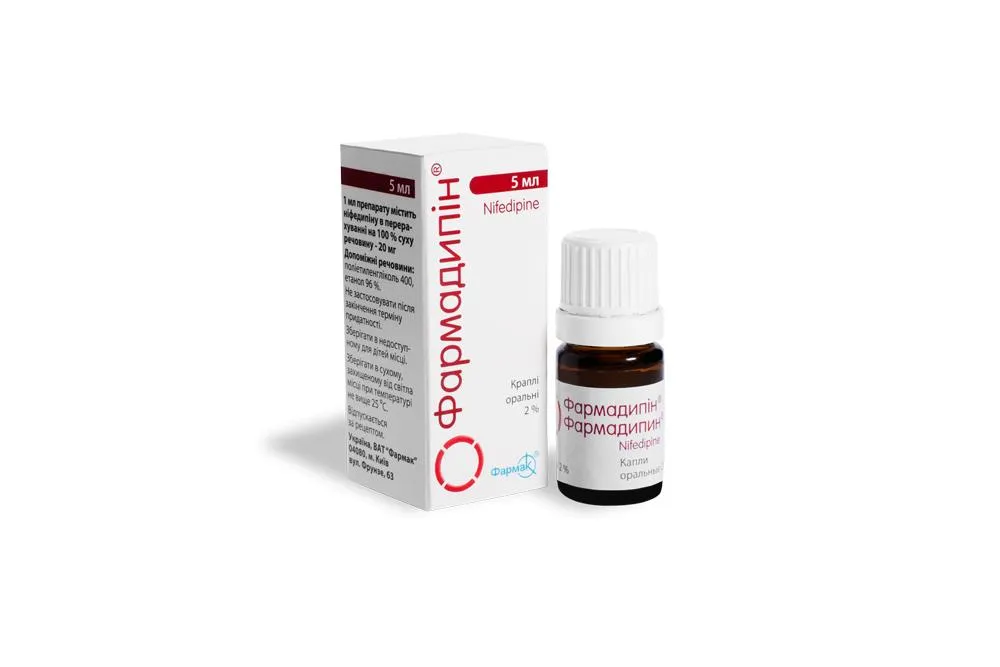
Farmadipin (Nifedipine) Oral Drops 2% 5 ml
Ingredients
Active ingredient: Nifedipine 2%
Dosage
Recommended dosage: Consult your healthcare provider for the appropriate dosage as it may vary based on individual circumstances.
Indications
Farmadipin oral drops are indicated for the treatment of hypertension (high blood pressure) and angina (chest pain).
Contraindications
Do not use Farmadipin oral drops if you:
- Are allergic to nifedipine or any other ingredients in the product.
- Have low blood pressure.
- Are pregnant or breastfeeding without medical advice.
Directions
How to use: Administer the drops orally as directed by your healthcare provider. Do not exceed the recommended dosage.
Scientific Evidence
Nifedipine, the active ingredient in Farmadipin oral drops, belongs to the class of medications known as calcium channel blockers. It works by relaxing the blood vessels, making it easier for the heart to pump blood and lower blood pressure. Studies have shown the efficacy of nifedipine in managing hypertension and angina, providing symptomatic relief and improving overall cardiovascular health.
Additional Information
It is essential to follow the prescribed dosage and recommendations provided by your healthcare provider when using Farmadipin oral drops. Inform your doctor about any other medications or supplements you are taking to avoid potential drug interactions. Regular monitoring of blood pressure and heart function may be necessary during treatment with nifedipine.
Research has highlighted the role of nifedipine in improving exercise tolerance and reducing the frequency of angina attacks in patients with coronary artery disease. The drug’s vasodilatory effects contribute to its effectiveness in managing cardiovascular conditions, making it a valuable option in the treatment of hypertension and angina.