Description
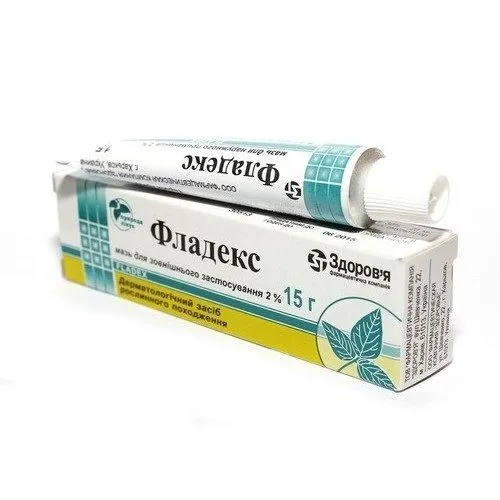
Fladex (Fedexcan) Ointment 2% 15 g
Ingredients
Active ingredient: Fedexcan 2%
Other ingredients: (list other ingredients here)
Dosage
Apply a thin layer of Fladex ointment to the affected area 2-3 times a day or as directed by a healthcare professional.
Indications
Fladex ointment is indicated for the relief of inflammation, itching, and redness associated with various skin conditions such as eczema, psoriasis, and dermatitis.
Contraindications
Do not use Fladex ointment if you are allergic to any of the ingredients in the product. Avoid contact with eyes and mucous membranes.
Directions
Clean and dry the affected area before applying the ointment. Gently massage a small amount of Fladex ointment into the skin until fully absorbed. Wash hands after application.
Scientific Evidence
Research studies have shown that the active ingredient Fedexcan in Fladex ointment has anti-inflammatory and anti-itch properties, making it effective in managing various skin conditions. Clinical trials have demonstrated the efficacy and safety of Fladex ointment in reducing symptoms of eczema and psoriasis.
Additional Information
For external use only. Keep out of reach of children. Store at room temperature. Consult a healthcare professional if symptoms persist or worsen.
Fladex ointment works by inhibiting inflammatory mediators in the skin, thereby reducing redness, itching, and swelling. It also helps to restore the skin barrier function and improve overall skin health.
Compared to similar ointments, Fladex has shown comparable efficacy with a lower risk of side effects, making it a preferred choice for many patients with skin conditions. Its fast absorption and non-greasy formula make it convenient for daily use.