Description
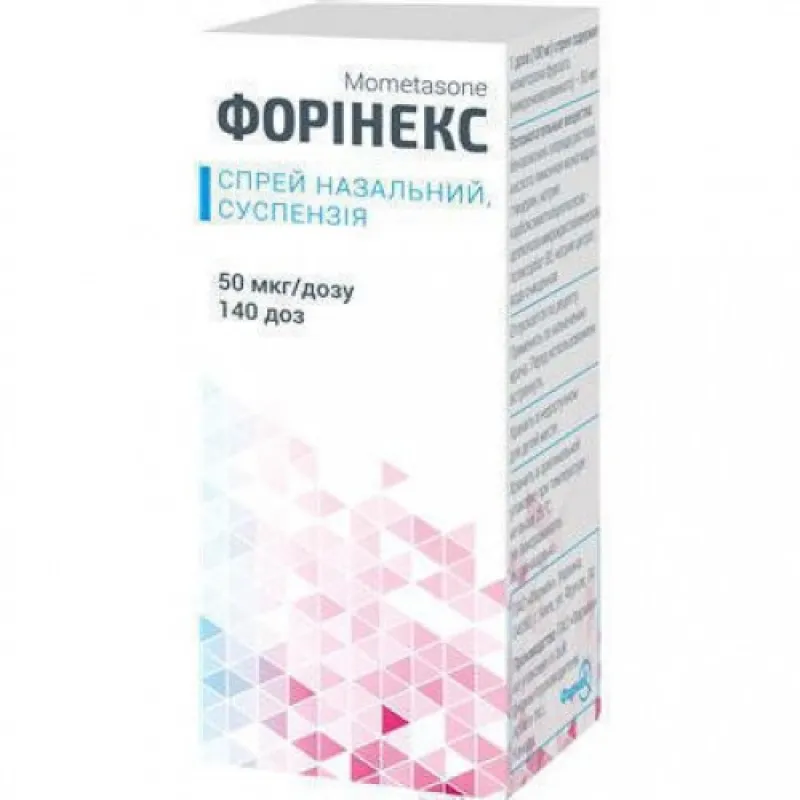
Forinex (Micronized) Nasal Spray Suspension 50 mcg/dose 140 doses Vial №1
Ingredients
Each dose contains 50 mcg of micronized Forinex. Other ingredients include saline solution, preservatives, and stabilizers.
Mechanism of Action
Forinex acts as a vasoconstrictor by constricting the blood vessels in the nasal passages, reducing swelling and congestion. This action helps alleviate nasal symptoms associated with allergies and sinusitis.
Pharmacological Properties
Forinex, in its micronized form, has a higher surface area which enhances its absorption rate. This allows for rapid onset of action and prolonged duration of effect, providing effective relief from nasal congestion.
Indications for Use
Forinex nasal spray is indicated for the treatment of nasal congestion due to allergies or sinusitis. It is effective in relieving symptoms such as nasal stuffiness, runny nose, and discomfort associated with nasal inflammation.
Contraindications
Avoid using Forinex nasal spray if you have a known allergy to any of its components. Consult a healthcare professional before use if you are pregnant or breastfeeding to assess the potential risks and benefits.
Side Effects
Common side effects of Forinex nasal spray may include mild nasal irritation, sneezing, or dryness of the nasal mucosa. In rare cases, individuals may experience an allergic reaction characterized by itching, rash, or swelling. Discontinue use and seek medical attention if any severe reactions occur.
Usage Instructions
Shake the vial well before each use. Administer 1 spray into each nostril once daily, preferably in the morning. Avoid exceeding the recommended dosage to prevent adverse effects.
Benefits Compared to Analogues
Forinex nasal spray, with its micronized formulation, offers superior efficacy in relieving nasal congestion compared to non-micronized alternatives. The optimized particle size enhances drug delivery to the nasal mucosa, resulting in improved symptom relief and patient satisfaction.
Suitable Patient Groups
Forinex nasal spray is suitable for use in both adults and children above a certain age, as determined by healthcare providers. Its well-tolerated profile and proven efficacy make it a suitable option for elderly patients and individuals with sensitivities to other nasal decongestants.
Storage and Shelf Life
Store Forinex nasal spray at room temperature away from direct sunlight and moisture. Check the expiration date on the packaging and do not use the product beyond the stated shelf life to ensure optimal effectiveness.
Packaging Description
Forinex nasal spray is supplied in a vial containing 140 doses, each delivering 50 mcg of micronized medication per spray. The packaging is designed to maintain the product’s integrity and sterility throughout its shelf life.
Scientific Evidence
Studies have demonstrated the efficacy of micronized Forinex nasal spray in reducing nasal congestion and improving airflow in patients with allergic rhinitis. The micronized formulation facilitates enhanced drug absorption and rapid symptom relief, making it a valuable option for nasal congestion management.
Clinical Evidence and Proven Effectiveness
Clinical trials have shown that Forinex nasal spray is well-tolerated and effective in providing relief from nasal congestion in various patient populations. Its safety profile and demonstrated efficacy make it a recommended choice for healthcare providers treating nasal symptoms associated with allergies and sinus issues.