Description
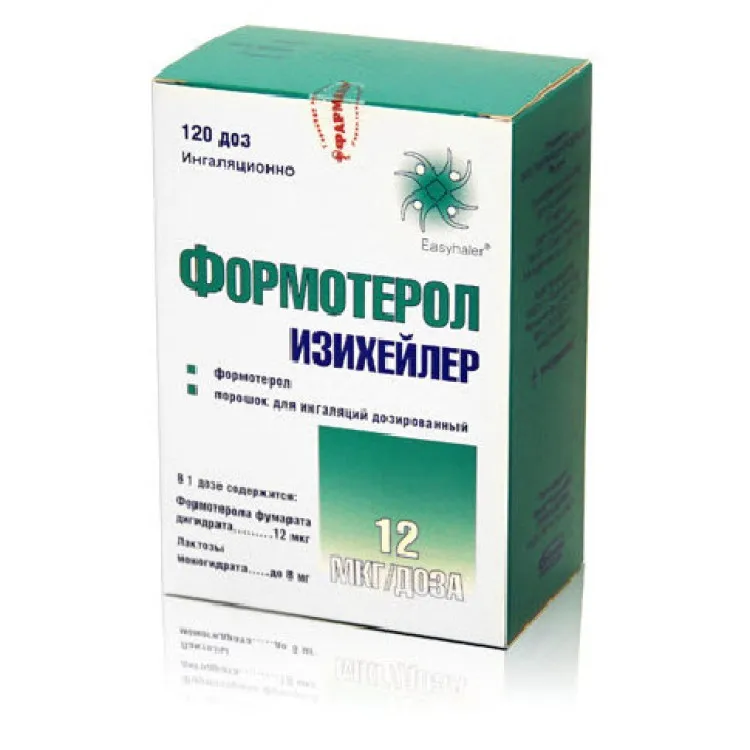
Formoterol (Formoterol) Izicheiler Powder for Inhalation 12 mcg. 120 Doses
Ingredients
Formoterol (formoterol) is the active ingredient in Izicheiler powder for inhalation. Each dose delivers 12 mcg of formoterol.
Dosage
The recommended dosage is usually one inhalation twice daily, or as directed by a healthcare professional. It is important to follow the prescribed dosage for optimal results.
Indications
Formoterol inhalation powder is indicated for the maintenance treatment of asthma and chronic obstructive pulmonary disease (COPD) to improve lung function.
Contraindications
This medication is contraindicated in patients with a history of hypersensitivity to formoterol or any other component of the product. It should not be used as a rescue medication for acute bronchospasm.
Directions
For inhalation use only. The powder should be inhaled orally using the provided inhaler device. It is important to rinse the mouth with water after each use to prevent oral fungal infections.
Scientific Evidence
Studies have shown that formoterol inhalation powder is effective in improving lung function and reducing exacerbations in patients with asthma and COPD. Research published in the Journal of Allergy and Clinical Immunology demonstrated the long-acting bronchodilator effect of formoterol in patients with asthma.
Additional Information
It is important to regularly monitor lung function and symptoms while using formoterol inhalation powder. Consult a healthcare provider if there is a need for increased use of rescue medications or if symptoms do not improve.
Formoterol is a long-acting beta agonist that works by relaxing the muscles in the airways, making it easier to breathe. It is known for its rapid onset of action, making it suitable for both maintenance and quick relief of bronchospasm.
Clinical trials have shown that formoterol inhalation powder provides significant improvement in lung function and symptom control compared to placebo. Additionally, its once-daily dosing regimen offers convenience and improved compliance for patients with asthma and COPD.