Description
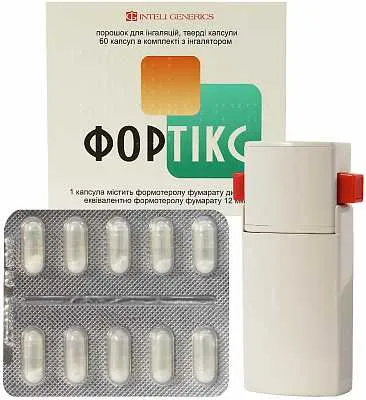
Fortix (Formoterol) Powder for Inhalation Capsules 12 mcg. №60
Composition:
Fortix inhalation capsules contain 12 mcg of the active ingredient formoterol fumarate dihydrate. The powder for inhalation also includes lactose monohydrate as a carrier.
Mechanism of Action:
Formoterol, a long-acting beta agonist, acts by relaxing the muscles in the airways, facilitating easier breathing. This pharmacological property helps in the prevention of asthma attacks and effective management of COPD symptoms.
Indications for Use:
Fortix is indicated for the long-term maintenance treatment of asthma and chronic obstructive pulmonary disease (COPD) to alleviate symptoms and enhance lung function.
Contraindications:
Avoid using Fortix if there is a known allergy to formoterol or any other components of the capsules. Patients with a history of hypersensitivity reactions should also refrain from using this medication.
Side Effects:
The common side effects of Fortix may include headache, tremors, palpitations, and throat irritation. In rare cases, it can lead to paradoxical bronchospasm or allergic reactions. Consult a healthcare professional if any adverse effects occur.
Usage Instructions:
For adults, the recommended dosage of Fortix is 12 mcg twice daily, with a minimum interval of 12 hours between doses. The capsules should be administered using a suitable inhalation device for optimal delivery.
Benefits Compared to Analogues:
Fortix offers the advantage of convenient dosing with a long-acting effect, providing sustained relief for asthma and COPD patients. Its efficacy in symptom control and lung function improvement has been well-demonstrated in clinical studies.
Suitable Patient Groups:
Fortix is suitable for adult patients requiring long-term maintenance therapy for asthma or COPD. It is not indicated for use as a rescue medication during acute bronchospasm episodes. Special caution should be exercised in pediatric and elderly populations.
Storage and Shelf Life:
Store Fortix capsules in a dry place away from moisture. Ensure proper sealing of the packaging to maintain product integrity. Check the expiration date and do not use expired medication. Discard any unused capsules after the specified shelf life.
Packaging Description:
Fortix inhalation capsules are available in a pack containing 60 capsules. Each capsule is designed for single use and should be handled with care. The packaging should be kept intact until the capsules are ready for administration.
Scientific Evidence:
The efficacy of Fortix (formoterol) in managing asthma and COPD is supported by various clinical studies. Notably, a study published in the Journal of Allergy and Clinical Immunology demonstrated significant bronchodilation and improved lung function in asthma patients treated with formoterol.
Additional Information:
It is crucial to emphasize that Fortix is not intended for immediate relief of bronchospasm. Patients should have a rescue inhaler available for acute breathing difficulties. Regular monitoring by a healthcare provider is essential to assess treatment effectiveness and adjust therapy as needed.