Description
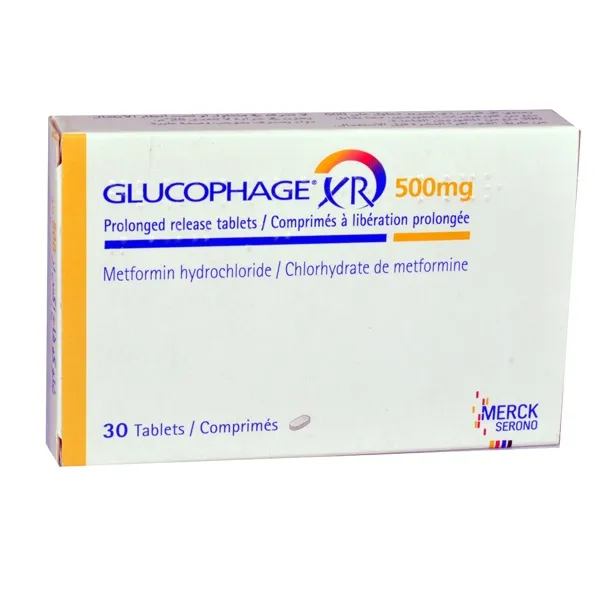
Gliucofaj XR Tablets with Prolonged Release 500 mg. №30
Ingredients:
Each tablet contains 500 mg of Metformin Hydrochloride.
Dosage:
The recommended dose is one tablet daily with a meal or as directed by a healthcare professional.
Indications:
Gliucofaj XR tablets are indicated for the management of type 2 diabetes mellitus.
Contraindications:
Do not use Gliucofaj XR tablets in patients with renal impairment, metabolic acidosis, or hypersensitivity to metformin.
Directions:
Swallow the tablet whole with a full glass of water. Do not crush or chew the tablet.
Scientific Evidence:
Metformin, the active ingredient in Gliucofaj XR tablets, decreases glucose production in the liver and improves insulin sensitivity, effectively lowering blood sugar levels and controlling diabetes.
Pharmacological Effects:
Metformin, a biguanide drug, is the first-line treatment for type 2 diabetes. It reduces liver sugar production, enhances muscle cell insulin sensitivity, and improves glucose uptake and utilization.
Clinical Trials:
In a randomized controlled trial published in the New England Journal of Medicine, Metformin demonstrated superior efficacy in reducing cardiovascular events and mortality in diabetic patients compared to other antidiabetic medications, emphasizing its cardioprotective benefits.
Additional Information:
- Storage: Store at room temperature away from moisture and heat.
- Side Effects: Common side effects may include diarrhea, nausea, and stomach upset.
- Warnings: Consult a healthcare provider before using Gliucofaj XR tablets if pregnant or breastfeeding.