Description
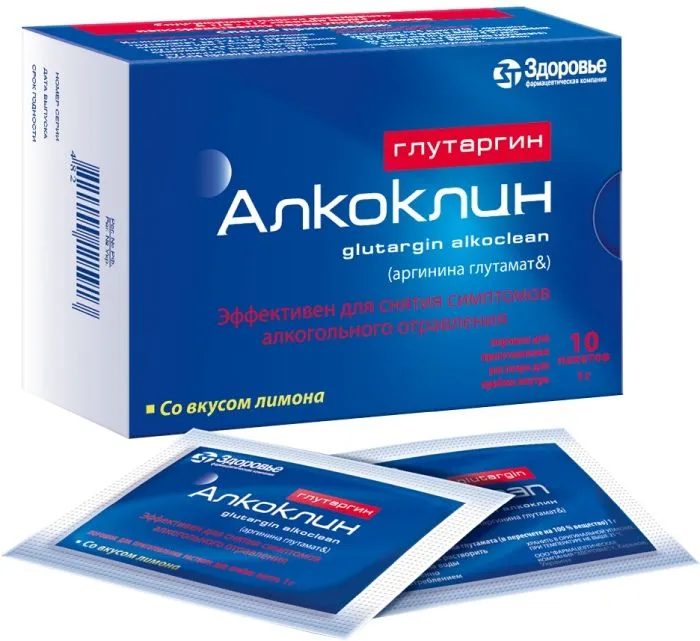
Glutargyn Alcolyn Powder 1g/3g. №10
Ingredients:
Glutamine 1g, Arginine 3g
Mechanism of Action:
Glutargyn Alcolyn Powder combines glutamine and arginine to support muscle recovery and promote protein synthesis. Glutamine aids in immune function and gut health, while arginine facilitates nitric oxide production, enhancing vasodilation and blood flow to muscles.
Pharmacological Properties:
The product’s formulation harnesses the synergistic effects of glutamine and arginine to optimize muscle metabolism, aid in muscle repair, and enhance exercise performance.
Indications for Use:
Glutargyn Alcolyn Powder is indicated for supporting muscle recovery and promoting protein synthesis, making it beneficial for individuals engaging in physical activities or fitness training.
Contraindications:
Avoid using Glutargyn Alcolyn Powder if pregnant or breastfeeding. It is advisable to consult a healthcare professional before use, especially if you have pre-existing medical conditions.
Side Effects:
While generally well-tolerated, some individuals may experience mild gastrointestinal discomfort. Discontinue use if adverse reactions occur.
Usage Instructions:
Take one sachet of Glutargyn Alcolyn Powder daily, dissolved in water. Do not exceed the recommended dosage to ensure safe and effective use.
Benefits Compared to Analogues:
Compared to similar products, Glutargyn Alcolyn Powder offers a balanced combination of glutamine and arginine, providing comprehensive support for muscle recovery and exercise performance.
Suitable Patient Groups:
This product is suitable for adults engaging in physical activities, athletes, and fitness enthusiasts looking to enhance their muscle recovery and performance. It is not recommended for pregnant or breastfeeding individuals.
Storage and Shelf Life:
Store Glutargyn Alcolyn Powder in a cool, dry place away from direct sunlight. Check the expiry date on the packaging and avoid using expired products.
Packaging Description:
The product is packaged in individual sachets for convenient daily use. Each box contains 10 sachets of Glutargyn Alcolyn Powder.
Clinical Evidence and Proven Effectiveness:
Studies have demonstrated the efficacy of glutamine and arginine supplementation in improving exercise performance, reducing muscle soreness, and supporting muscle growth. A study published in the Journal of Strength and Conditioning Research reported enhanced muscle recovery and performance in athletes using a combination of glutamine and arginine.
Additional Information:
Glutargyn Alcolyn Powder is designed to complement your fitness regimen by providing essential amino acids for muscle repair and growth. Prior consultation with a healthcare provider is recommended before initiating any new supplement regimen, particularly if you have underlying health conditions or are taking medications that may interact with the product.