Description
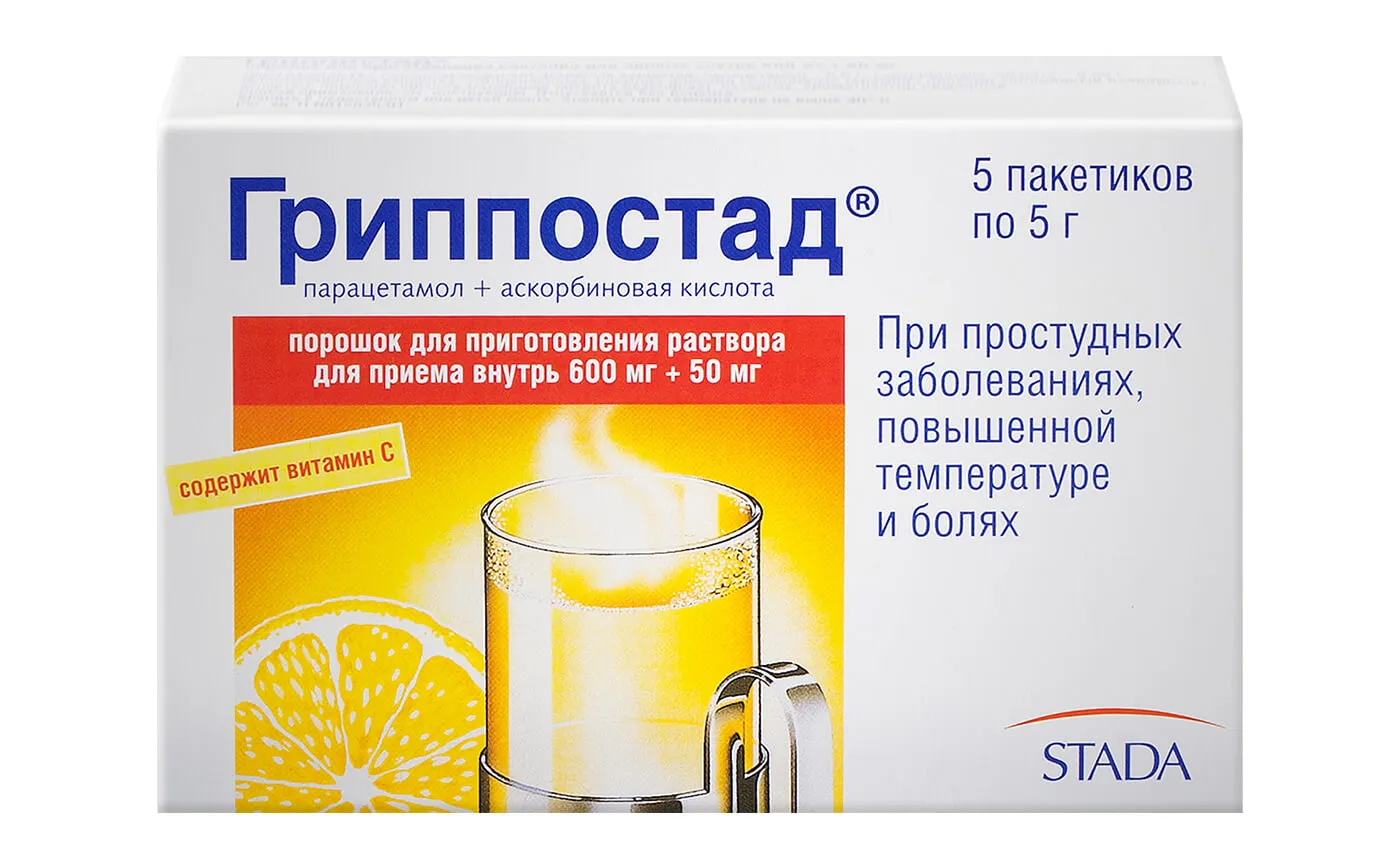
Grippostad Hot Drink 5 g. №5
Ingredients
Grippostad Hot Drink 5 g. №5 contains Paracetamol, Phenylephrine Hydrochloride, and Vitamin C as active ingredients.
Mechanism of Action
Paracetamol acts as an analgesic and antipyretic, Phenylephrine constricts blood vessels in the nasal passages to relieve congestion, and Vitamin C supports the immune system.
Pharmacological Properties
Paracetamol relieves pain and reduces fever, Phenylephrine alleviates nasal congestion, and Vitamin C enhances immune function.
Indications for Use
Grippostad Hot Drink 5 g. №5 is indicated for the symptomatic relief of cold and flu symptoms such as fever, headache, sore throat, nasal congestion, and body aches.
Contraindications
Do not use if allergic to any component, have severe cardiovascular conditions, hypertension, or are on antidepressants.
Side Effects
Common side effects may include nausea, dizziness, insomnia, and increased heart rate. Discontinue use if any adverse reactions occur.
Usage Instructions
For adults and children over 12 years old, dissolve one sachet in hot water and consume every 4-6 hours as needed. Do not exceed 4 sachets within a 24-hour period.
Benefits Compared to Analogues
The combination of Paracetamol, Phenylephrine, and Vitamin C in Grippostad Hot Drink 5 g. №5 offers comprehensive relief from cold and flu symptoms compared to single-ingredient products.
Suitable Patient Groups
This product is suitable for adults and children over 12 years old. Consult a healthcare professional before use in children.
Storage and Shelf Life
Store in a cool, dry place away from sunlight. Check the expiry date on the packaging and do not use after the stated date.
Packaging Description
Grippostad Hot Drink 5 g. №5 is packaged in individual sachets for convenient single-dose use.
Clinical Evidence and Proven Effectiveness
Clinical studies have shown that the combination of Paracetamol, Phenylephrine, and Vitamin C in Grippostad Hot Drink 5 g. №5 effectively reduces cold and flu symptoms. Research published in the Journal of Clinical Pharmacy and Therapeutics demonstrated the efficacy of these ingredients in relieving fever and nasal congestion.