Description
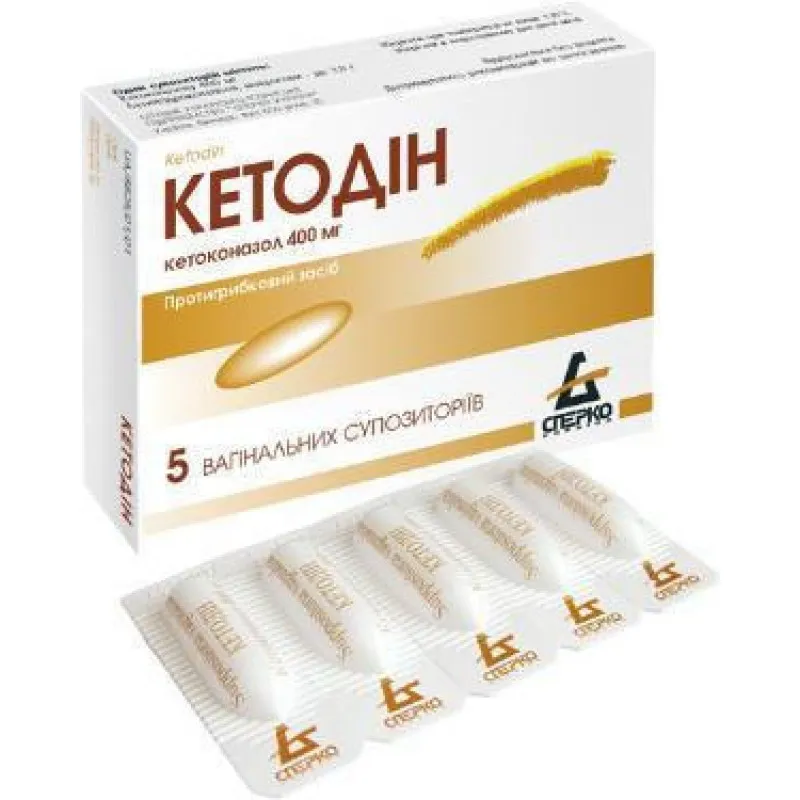
Ketodyn (Ketoconazole) Vaginal Suppositories 400 mg. №5
Ingredients
Each Ketodyn vaginal suppository contains 400 mg of ketoconazole, an antifungal agent effective against a wide range of fungi and yeasts.
Mechanism of Action
Ketoconazole exerts its antifungal effects by inhibiting the synthesis of ergosterol, a key component of fungal cell membranes. This disruption leads to increased membrane permeability and ultimately cell death.
Pharmacological Properties
Ketoconazole demonstrates fungistatic or fungicidal activity against various pathogenic fungi, including Candida species. It is well-absorbed following intravaginal administration, allowing for targeted treatment of vaginal infections.
Indications for Use
Ketodyn is specifically indicated for the treatment of vaginal yeast infections caused by Candida species. Additionally, it can be employed for the prevention of recurrent fungal infections in susceptible individuals.
Contraindications
- Patients with known hypersensitivity to ketoconazole or any other components of Ketodyn should avoid its use.
- Usage of Ketodyn during pregnancy is generally contraindicated unless deemed essential and under the guidance of a healthcare provider.
Side Effects
Common side effects associated with Ketodyn may include local irritation, burning, or itching at the application site. In rare cases, systemic adverse reactions such as allergic dermatitis or hepatotoxicity may occur.
Usage Instructions
For optimal therapeutic outcomes, insert one suppository into the vagina before bedtime using the provided applicator. Hands should be washed before and after administration. It is crucial to complete the full prescribed course of treatment even if symptoms resolve sooner.
Benefits Compared to Analogues
Ketodyn offers a potent antifungal effect with a convenient once-daily dosing regimen. Its localized action minimizes systemic exposure, reducing the likelihood of systemic side effects compared to oral antifungal agents.
Suitable Patient Groups
Ketodyn is generally safe for use in adult women, including elderly individuals. However, caution should be exercised in pregnant patients, and its use in pediatric populations has not been extensively studied.
Storage and Shelf Life
Ketodyn should be stored at controlled room temperature away from moisture and heat. The shelf life of the product is typically indicated on the packaging and should be adhered to for optimal efficacy.
Packaging Description
Ketodyn is typically packaged in blister packs containing 5 vaginal suppositories. The packaging should be intact upon purchase, and any signs of tampering should be reported to the dispensing pharmacy.