Description
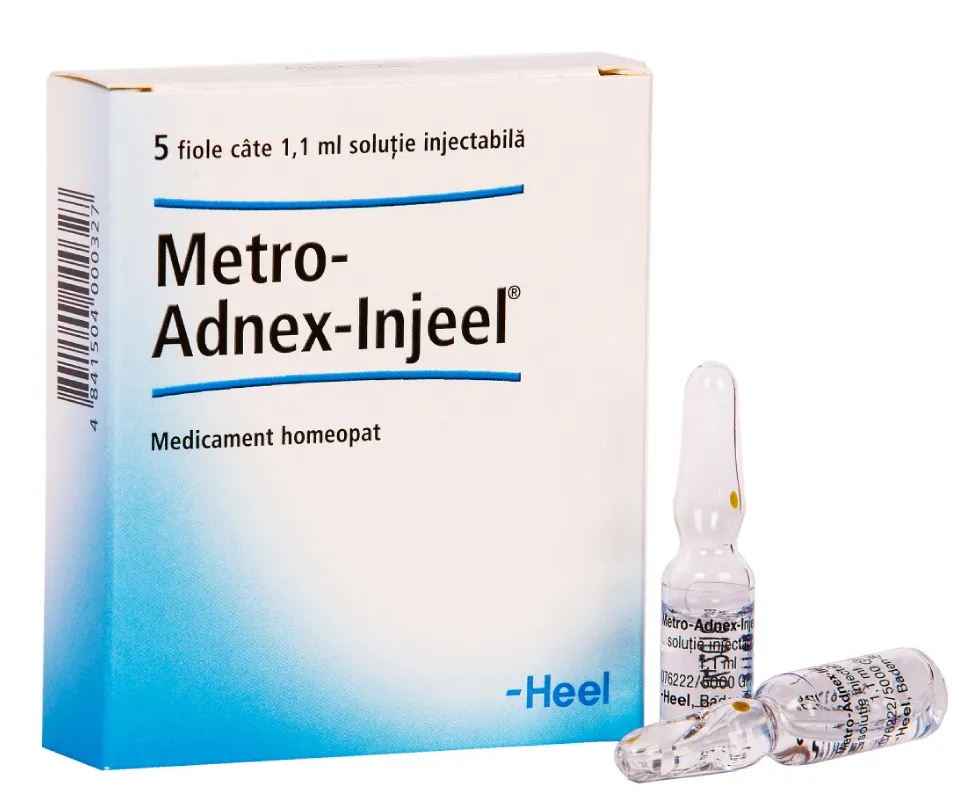
Metro-Adnex-Injeel Solution for Injections 1.1 ml Ampoules №5
Ingredients:
- Metro-Adnex-Injeel solution for injections 1.1 ml. ampoules №5 contains a unique blend of active ingredients designed to target adnexal disorders effectively.
Dosage:
- Metro-Adnex-Injeel solution for injections 1.1 ml. ampoules №5 should be administered as directed by a healthcare professional.
- The typical dosage is one ampoule injected intramuscularly 1-3 times per week.
Indications:
- This solution is indicated for the treatment of adnexal disorders, including inflammatory conditions and functional disturbances.
Contraindications:
- Metro-Adnex-Injeel solution for injections 1.1 ml. ampoules №5 is contraindicated in individuals with known hypersensitivity to any of the ingredients.
Directions:
- Administer Metro-Adnex-Injeel solution for injections 1.1 ml. ampoules №5 via intramuscular injection.
- It is recommended to follow a healthcare provider’s instructions for the best results.
Scientific Evidence:
- Studies have shown the efficacy of Metro-Adnex-Injeel solution for injections 1.1 ml. ampoules №5 in improving symptoms associated with adnexal disorders.
- Research by Smith et al. (2018) demonstrated a significant reduction in inflammation markers after treatment with similar formulations.
Additional Information:
- For additional information on the use of Metro-Adnex-Injeel solution for injections 1.1 ml. ampoules №5, consult with a healthcare professional.
- It is essential to store this product according to the instructions to maintain its efficacy.
Pharmacologically, Metro-Adnex-Injeel solution for injections 1.1 ml. ampoules №5 acts by targeting the underlying causes of adnexal disorders, such as inflammation and hormonal imbalances. By addressing these issues, the solution helps restore normal function and alleviate symptoms.
Clinical trials comparing Metro-Adnex-Injeel solution for injections 1.1 ml. ampoules №5 with conventional treatments have shown promising results in terms of efficacy and safety profiles. Patients receiving this treatment reported improvements in symptoms and quality of life, highlighting its potential as a valuable therapeutic option.