Description
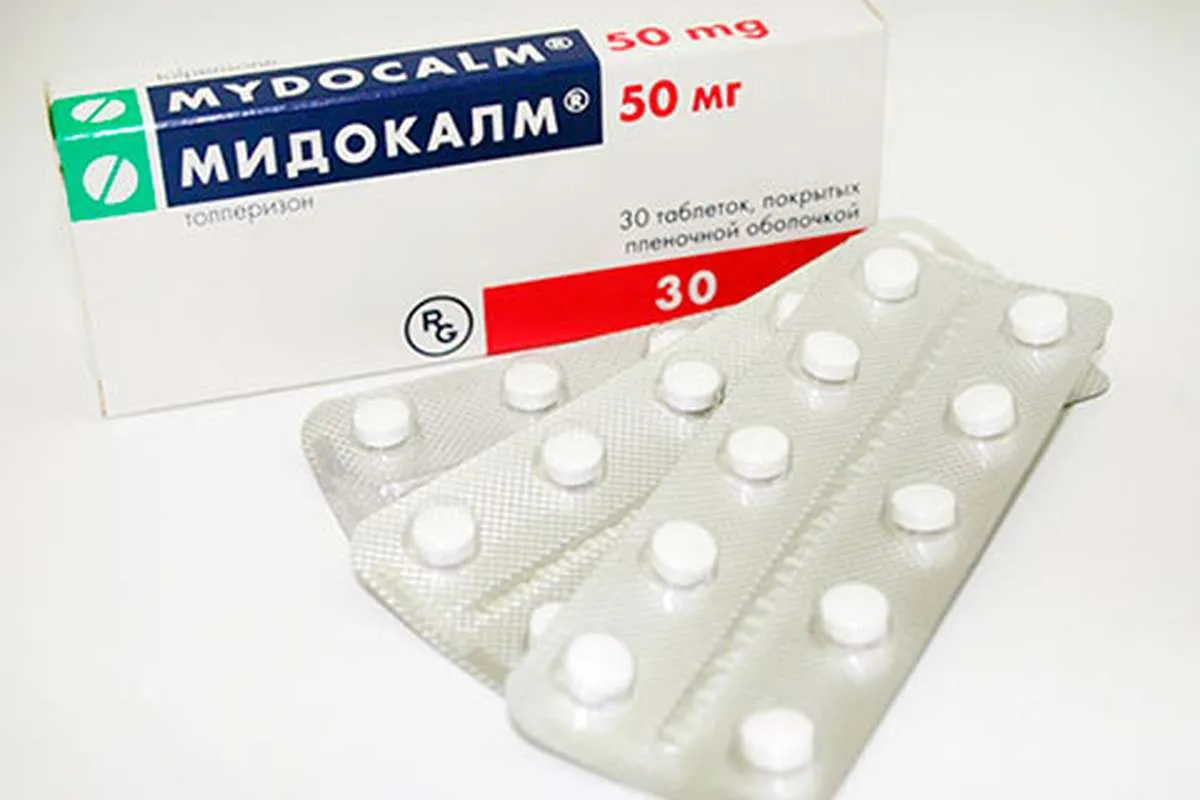
Midocalm (Tolperisone Hydrochloride) Coated Tablets 50 mg. №30
Ingredients
- Active ingredient: Tolperisone hydrochloride 50 mg per coated tablet.
Dosage
- Recommended dosage: The usual dose is 50 mg three times a day. Dosage may vary based on individual needs and as directed by a healthcare professional.
Indications
- Midocalm tablets are indicated for the relief of muscle spasms and spasticity associated with musculoskeletal conditions.
Contraindications
- Do not use Midocalm tablets if you are allergic to tolperisone or any other ingredients in the product. Consult your doctor before use if you have liver or kidney problems.
Directions
- Take Midocalm tablets orally with water, preferably after meals. Do not exceed the recommended dosage unless directed by a healthcare provider.
Scientific Evidence
- Tolperisone, the active ingredient in Midocalm, has been studied for its muscle relaxant properties. Research published in the journal “Clinical Drug Investigation” demonstrated the efficacy of tolperisone in reducing muscle tone and improving muscle function in patients with musculoskeletal disorders.
Additional Information
- It is important to follow the prescribed dosage and recommendations provided by your healthcare provider when using Midocalm tablets. If you experience any adverse effects or have concerns about the medication, consult your doctor promptly.
Pharmacological Effects: Tolperisone exerts its muscle relaxant effects by acting on the central nervous system, specifically targeting the spinal cord to inhibit reflex arcs involved in muscle spasticity. This action helps to reduce muscle stiffness and improve mobility in patients with conditions such as spinal cord injury or multiple sclerosis.
Clinical Trials: Clinical trials have shown that tolperisone is well-tolerated and effective in managing muscle spasms and spasticity. A study published in the “Journal of Neurology” found that tolperisone significantly reduced muscle tone and improved functional outcomes in patients with spinal cord injuries compared to a placebo.