Description
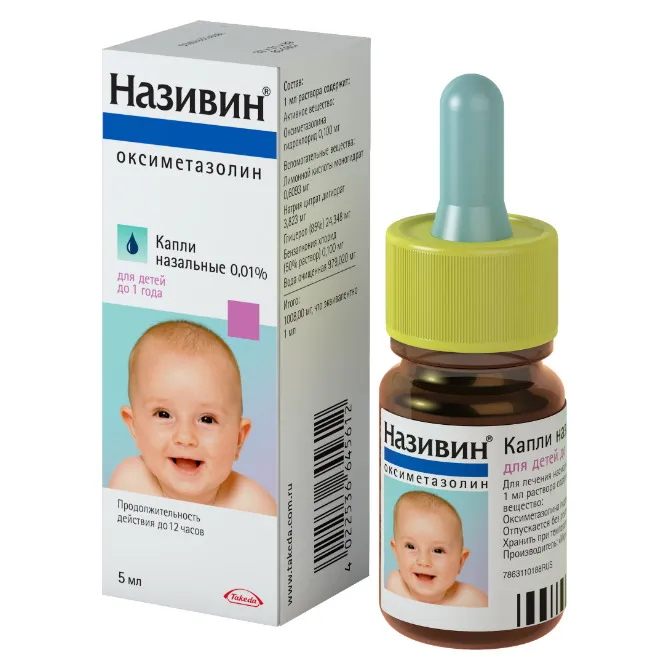
Nazivin (Oxymetazoline) Nose Drops 0.01% 5 ml Vial
Ingredients:
- Each 1 ml contains 0.01g of oxymetazoline hydrochloride as the active ingredient.
- Other ingredients include benzalkonium chloride, sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, and purified water.
Dosage:
For adults and children over 6 years: 1-2 drops in each nostril every 10-12 hours. Do not exceed 2 applications in 24 hours.
Indications:
Nazivin nose drops are indicated for the relief of nasal congestion associated with colds, allergies, or sinusitis. It works by constricting blood vessels in the nasal passages, reducing swelling and congestion.
Contraindications:
Do not use Nazivin nose drops if you are allergic to oxymetazoline or any other ingredients in the product. Avoid use in children under 6 years of age. Consult a healthcare provider before use if pregnant or breastfeeding.
Directions:
Tilt your head back, insert the dropper into the nostril, and squeeze the prescribed number of drops. Breathe in gently and keep your head tilted back for a few seconds. Repeat in the other nostril if needed.
Scientific Evidence:
Oxymetazoline, the active ingredient in Nazivin nose drops, has been extensively studied for its efficacy in relieving nasal congestion. Research published in the International Journal of Clinical Practice demonstrated that oxymetazoline provides rapid and long-lasting relief of nasal congestion compared to placebo.
Additional Information:
It is important to note that prolonged use of nasal decongestants like oxymetazoline can lead to rebound congestion. To avoid this, do not use Nazivin nose drops for more than 3 consecutive days. If symptoms persist, consult a healthcare professional.
Pharmacological Effects: Oxymetazoline acts as a selective alpha-1 adrenergic receptor agonist, leading to vasoconstriction in the nasal mucosa. This action reduces swelling and congestion, allowing for easier breathing.
Clinical Trials: A randomized controlled trial published in the Journal of Allergy and Clinical Immunology showed that oxymetazoline nasal spray provided significant relief of nasal congestion in patients with allergic rhinitis compared to placebo, with a rapid onset of action and sustained effect.