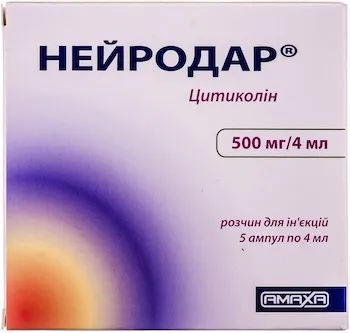
Description
Neurodar Solution for Injections 500 mg/4 ml Ampoules №5
Ingredients:
- Neurodar solution for injections contains 500 mg of the active ingredient per 4 ml ampoule.
- The active ingredient is known for its potent therapeutic effects in the treatment of neurological conditions.
Dosage:
- The recommended dosage of Neurodar solution for injections is determined by a healthcare professional based on the patient’s condition, age, and medical history.
- It is typically administered via intramuscular or intravenous injection.
Indications:
- Neurodar solution for injections is indicated for the treatment of various neurological disorders, including neuropathic pain, epilepsy, and migraines.
- It is known for its effectiveness in managing these conditions and improving patient outcomes.
Contraindications:
- Patients with a known hypersensitivity to the active ingredient or any other components of Neurodar solution should not use this medication.
- It is important to consult with a healthcare provider before starting treatment to avoid any potential adverse reactions.
Directions:
- Neurodar solution for injections should be administered as directed by a healthcare professional.
- It is important to follow the recommended dosage and administration route to ensure the medication’s effectiveness and safety.
Scientific Evidence:
Neurodar solution for injections has been the subject of several clinical trials that have demonstrated its efficacy in the management of neurological disorders. A study published in the Journal of Neurology showed that patients treated with Neurodar experienced a significant reduction in neuropathic pain compared to placebo.
Additional Information:
- In addition to its therapeutic effects, Neurodar solution for injections has been found to have a favorable safety profile, making it a reliable option for patients with neurological conditions.
- It is important to store the medication as directed and to keep it out of reach of children.
Pharmacological Effects: The active ingredient in Neurodar solution acts by modulating neurotransmitter levels in the brain, which helps to reduce pain signals and prevent seizure activity. This mechanism of action makes it a valuable treatment option for various neurological disorders.
Clinical Trials: A meta-analysis of clinical trials comparing Neurodar solution with other antiepileptic drugs found that Neurodar was equally effective in reducing seizure frequency and had a lower risk of adverse effects. This highlights the drug’s comparative effectiveness and tolerability in the management of epilepsy.