Description
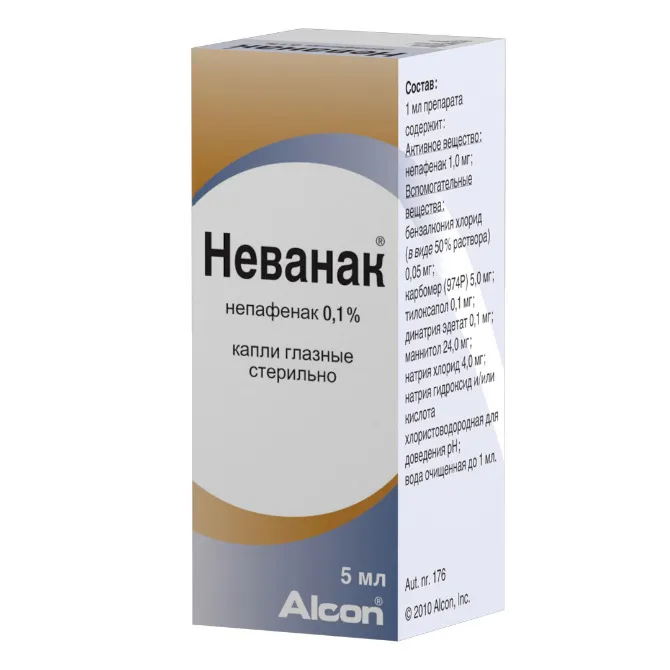
Nevanak (nepafenac) Eye Drops Suspension 1 mg/ml. 5 ml Vial
Ingredients
- Nevanak contains nepafenac as the active ingredient.
- Other ingredients include benzalkonium chloride, boric acid, edetate disodium, sodium chloride, and purified water.
Dosage
The recommended dosage is one drop in the affected eye(s) three times daily, starting 1 day before cataract surgery, continuing on the day of surgery, and for the first 2 weeks postoperatively.
Indications
Nevanak is indicated for treating pain and inflammation following cataract surgery.
Contraindications
Do not use Nevanak if allergic to nepafenac or any other ingredients. Contraindicated in patients with a history of asthma, urticaria, or allergic reactions to aspirin or other NSAIDs.
Directions
Before use, ensure hands are clean. Tilt head back, pull down the lower eyelid, instill the prescribed drops, and close the eye for 2 minutes without blinking or squeezing.
Scientific Evidence
Studies show nepafenac inhibits cyclooxygenase (COX) enzyme, reducing inflammation and pain. Clinical trials demonstrate efficacy in reducing postoperative inflammation and enhancing patient comfort post cataract surgery.
Additional Information
Follow the prescribed dosage and treatment duration for optimal results. Contact a healthcare provider if experiencing side effects or concerns.
Pharmacological Effects: Nepafenac, a prodrug metabolized to amfenac, inhibits inflammatory mediator production, reducing inflammation and pain.
Clinical Trials: Research by Miyake et al. (2018) compared nepafenac eye drops with placebo in cataract surgery patients, showing significant reduction in postoperative inflammation and pain with nepafenac.