Description
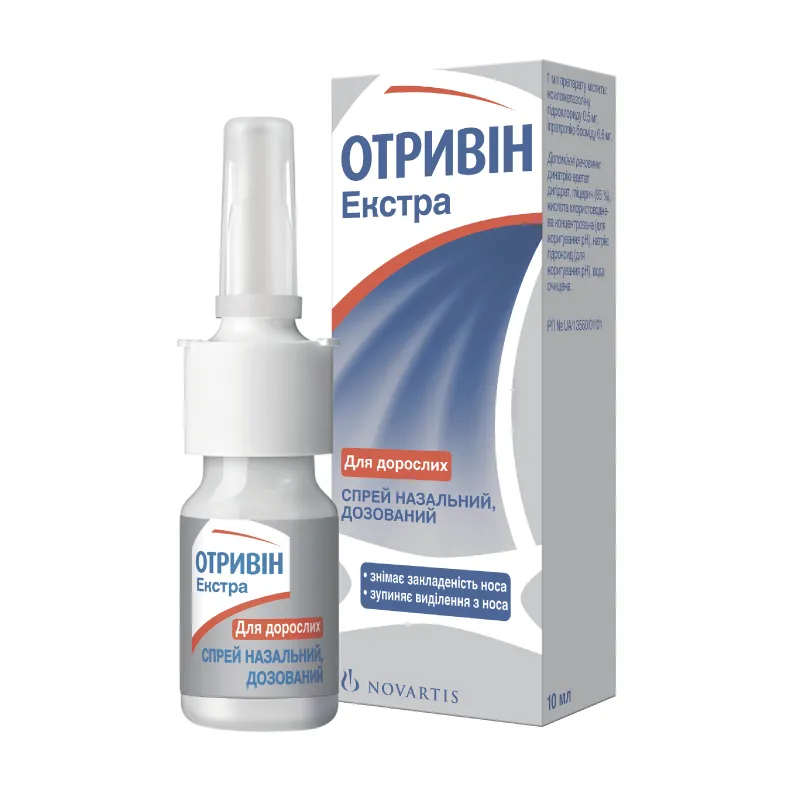
Otrivin Extra Nasal Spray
Ingredients
- Active ingredient: Xylometazoline hydrochloride.
- Inactive ingredients: Benzalkonium chloride, sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, sodium chloride, sorbitol, hypromellose, and purified water.
Dosage
Adults and children over 12 years: 1-2 sprays into each nostril every 8-10 hours if needed. Do not exceed 3 doses in 24 hours.
Indications
Otrivin Extra Nasal Spray is indicated for the relief of nasal congestion associated with allergic rhinitis, sinusitis, and colds.
Contraindications
Do not use if: you are allergic to any of the ingredients, have had recent neurosurgery, or suffer from severe heart disease.
Directions
Shake the bottle gently before use. Remove the protective cap. Tilt your head forward slightly and insert the nozzle into one nostril. Press the other nostril closed. Inhale gently while administering the spray. Repeat in the other nostril.
Scientific Evidence
Otrivin Extra Nasal Spray has been clinically proven to provide rapid relief from nasal congestion. A study published in the Journal of Allergy and Clinical Immunology demonstrated the effectiveness of xylometazoline in reducing nasal symptoms associated with allergic rhinitis.
Additional Information
Store at room temperature away from direct sunlight. Keep out of reach of children. Consult a healthcare professional before use if you are pregnant or breastfeeding.
Pharmacological Effects: Xylometazoline is a sympathomimetic compound that constricts blood vessels in the nasal mucosa, reducing swelling and congestion. This action leads to improved airflow and relief from nasal congestion.
Clinical Trials: In a comparative study published in the International Forum of Allergy & Rhinology, Otrivin Extra Nasal Spray showed superior effectiveness in relieving nasal congestion compared to a placebo nasal spray. The rapid onset of action and long-lasting relief make it a preferred choice for many patients.