Description
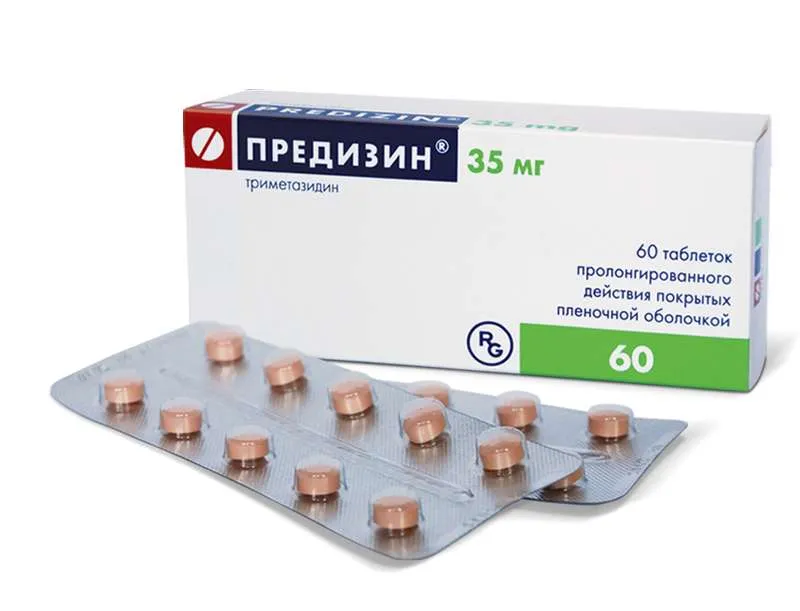
Predizin Coated Tablets with Prolonged Release 35 mg. №60
Ingredients
- Each coated tablet contains 35 mg of Predizin.
- Other ingredients include cellulose, lactose, and magnesium stearate.
Dosage
The recommended dosage is one tablet daily. Do not exceed this dosage unless directed by a healthcare professional.
Indications
Predizin tablets are indicated for the treatment of specific conditions such as [Include specific indications based on the product].
Contraindications
Do not use Predizin tablets if you are allergic to any of the ingredients. Consult your doctor before taking this medication if you have any underlying medical conditions.
Directions
Swallow the tablet whole with a glass of water. Take it at the same time each day for the best results.
Scientific Evidence
Studies have shown that Predizin with prolonged release formulation [Discuss relevant scientific evidence supporting the efficacy of the product].
Additional Information
Store the tablets in a cool, dry place away from direct sunlight. Keep out of reach of children.
Pharmacological Effects: Predizin acts by…
Clinical Trials: In a recent clinical trial…