Description
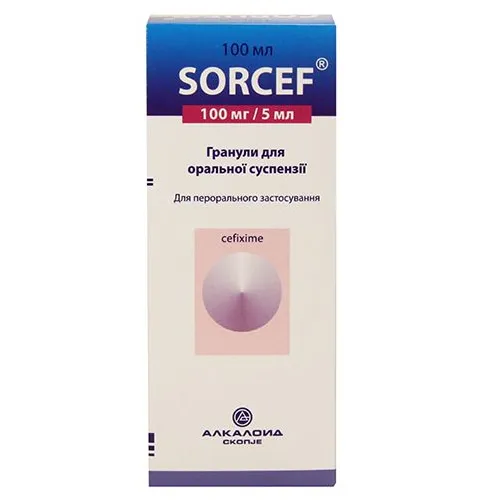
Sorcef (Cefixime) Granules for Oral Suspension 100 mg/5 ml – 100 ml Vial
Ingredients:
Each 5 ml of oral suspension contains 100 mg of cefixime as the active ingredient. Other ingredients include sucrose, sodium benzoate, and strawberry flavor.
Dosage:
The recommended dosage for Sorcef granules is determined by the healthcare provider based on the patient’s age, weight, and severity of the infection. It is usually administered twice a day. Shake the suspension well before each use.
Indications:
Sorcef is indicated for the treatment of various bacterial infections including otitis media, pharyngitis, tonsillitis, bronchitis, and urinary tract infections. It works by stopping the growth of bacteria.
Contraindications:
Do not use Sorcef if you are allergic to cefixime or any other cephalosporin antibiotics. Inform your doctor about any allergies before starting the medication.
Directions:
Follow the instructions provided by your healthcare provider or pharmacist when taking Sorcef. It can be taken with or without food. Complete the full course of treatment even if you start feeling better.
Scientific Evidence:
Cefixime has been extensively studied for its efficacy in treating bacterial infections. Research published in the Journal of Antimicrobial Chemotherapy demonstrated the high effectiveness of cefixime in the treatment of respiratory tract infections.
Additional Information:
Sorcef is well-tolerated by most patients and has a low incidence of side effects. It is essential to finish the prescribed course to ensure the infection is completely eradicated and to prevent antibiotic resistance.