Description
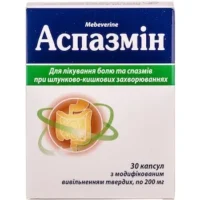
Spark (Mebeverin) Hard Capsules with Prolonged Release 200 mg. №30
Ingredients:
Each hard capsule contains 200 mg of mebeverine hydrochloride.
Dosage:
The recommended dosage is one capsule taken orally twice daily, preferably before meals.
Indications:
Spark (Mebeverin) is indicated for the symptomatic treatment of irritable bowel syndrome (IBS) characterized by abdominal pain, bloating, and altered bowel habits.
Contraindications:
Do not use Spark (Mebeverin) if you are allergic to mebeverine or any other ingredients in the product. Consult your healthcare provider before use if you are pregnant, breastfeeding, or have severe liver or kidney impairment.
Directions:
Swallow the capsule whole with a glass of water. Do not crush or chew the capsule. Follow the dosage instructions provided by your healthcare provider.
Scientific Evidence:
Mebeverine, the active ingredient in Spark capsules, acts as a musculotropic antispasmodic agent that relieves smooth muscle spasm in the gastrointestinal tract. Studies have shown that mebeverine effectively reduces abdominal pain and discomfort associated with IBS without affecting normal gut motility.
Research published in the “Journal of Gastroenterology” demonstrated the efficacy of mebeverine in improving IBS symptoms compared to a placebo, highlighting its role as a first-line treatment option for IBS patients.
Additional Information:
- Storage: Store Spark (Mebeverin) capsules in a cool, dry place away from direct sunlight.
- Side Effects: Common side effects may include dizziness, headache, and gastrointestinal disturbances. Consult your doctor if any adverse reactions occur.
- Interactions: Inform your healthcare provider about all medications you are taking, as mebeverine may interact with certain drugs.