Description
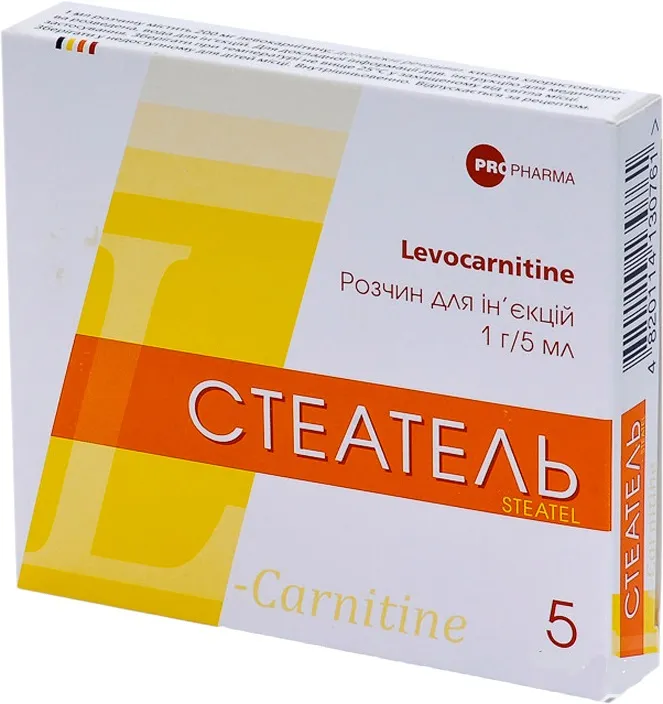
Steatel (levocarnitine) Solution for Injections 1 g/5 ml Ampoules 5 ml №5
Ingredients:
- Each 5 ml ampoule contains 1 g of levocarnitine.
Dosage:
- The usual dosage is determined by a healthcare provider and administered via intravenous or intramuscular injection.
Indications:
- Steatel injections are indicated for the treatment of primary and secondary carnitine deficiency.
- Carnitine deficiency can lead to symptoms such as muscle weakness, fatigue, and cardiac complications.
Contraindications:
- Do not use Steatel injections if you are allergic to levocarnitine or any other ingredients in the solution.
- Consult with a healthcare provider before use if you have any underlying medical conditions.
Directions:
- Administer the injection as directed by a healthcare professional.
- Do not self-administer without proper medical guidance.
Scientific Evidence:
- Levocarnitine has been extensively studied for its efficacy in treating carnitine deficiency.
- Research published in the Journal of Inherited Metabolic Disease demonstrated the positive impact of levocarnitine supplementation in improving symptoms and biochemical markers in patients with primary carnitine deficiency.
Additional Information:
- Pharmacological Effects: Levocarnitine plays a crucial role in fatty acid metabolism, facilitating the transport of long-chain fatty acids into the mitochondria for energy production.
- Clinical Trials: A randomized controlled trial published in the Journal of Clinical Investigation showed that levocarnitine supplementation improved exercise tolerance and muscle function in patients with secondary carnitine deficiency, highlighting its therapeutic potential.