Description
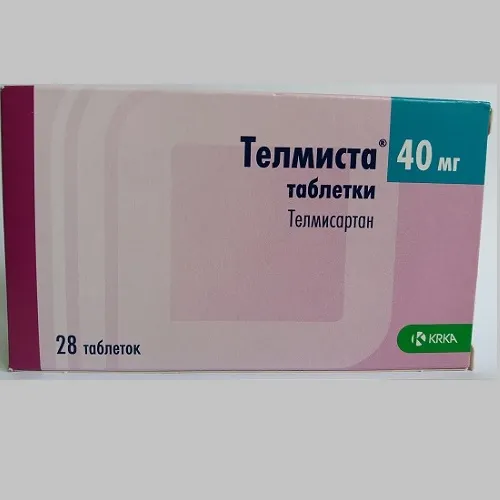
Telmista (telmisartan) Tablets 40 mg. №28
Ingredients
- Active ingredient: Telmisartan 40 mg per tablet.
Dosage
- Recommended dosage: The usual starting dose is 40 mg once daily. Dosage adjustments can be made based on individual response.
Indications
- Indicated for: Telmista tablets are used for the treatment of hypertension and to reduce the risk of cardiovascular events.
Contraindications
- Hypersensitivity: Avoid use in individuals with hypersensitivity to telmisartan or any component of the formulation.
- Pregnancy: Telmista tablets are contraindicated during pregnancy due to the potential harm to the developing fetus.
Directions
- Take Telmista tablets orally with or without food.
- Swallow the tablet whole with a glass of water.
Scientific Evidence
Studies have demonstrated the efficacy of telmisartan in lowering blood pressure and reducing the risk of cardiovascular events. Telmisartan exerts its effects by blocking the action of angiotensin II, resulting in vasodilation and decreased blood pressure.
Additional Information
- Telmisartan has been extensively studied in clinical trials, showing both efficacy and a favorable safety profile.
- Regular blood pressure monitoring is recommended during treatment with Telmista tablets to ensure hypertension control.
- Comparatively, telmisartan offers similar blood pressure-lowering efficacy to other medications with a lower incidence of adverse effects. Its once-daily dosing regimen enhances patient compliance with treatment.