Description
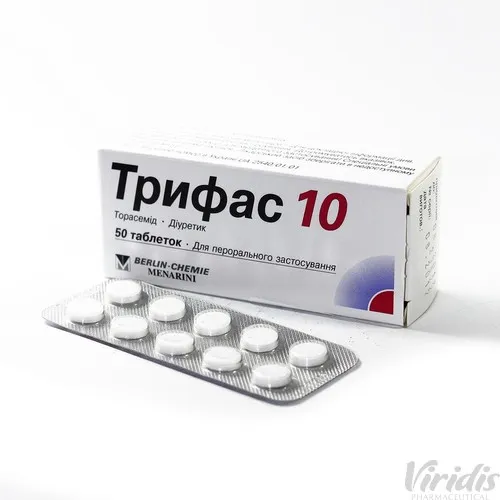
Trifas 10 (Torasemide) Tablets 10 mg. №50
Ingredients:
- Each tablet contains 10 mg of Torasemide.
Dosage:
- The usual recommended dose is 10 mg once daily.
Indications:
- Trifas 10 tablets are indicated for the treatment of hypertension and edema associated with congestive heart failure.
Contraindications:
- Do not use Trifas 10 if you are allergic to Torasemide or if you have anuria.
Directions:
- Take Trifas 10 tablets orally with or without food as directed by your doctor.
Scientific Evidence:
Studies have shown that Torasemide, the active ingredient in Trifas 10 tablets, is effective in the management of hypertension and heart failure. Torasemide works by inhibiting the reabsorption of sodium and chloride in the kidneys, leading to increased urine production and reduced fluid retention.
Additional Information:
- It is important to monitor electrolyte levels regularly while taking Trifas 10 tablets, as Torasemide can cause imbalances in potassium, sodium, and magnesium levels.
- Trifas 10 tablets have been well-tolerated in clinical trials, with common side effects including dizziness, headache, and dehydration.
- Report any severe side effects to your doctor promptly.