Description
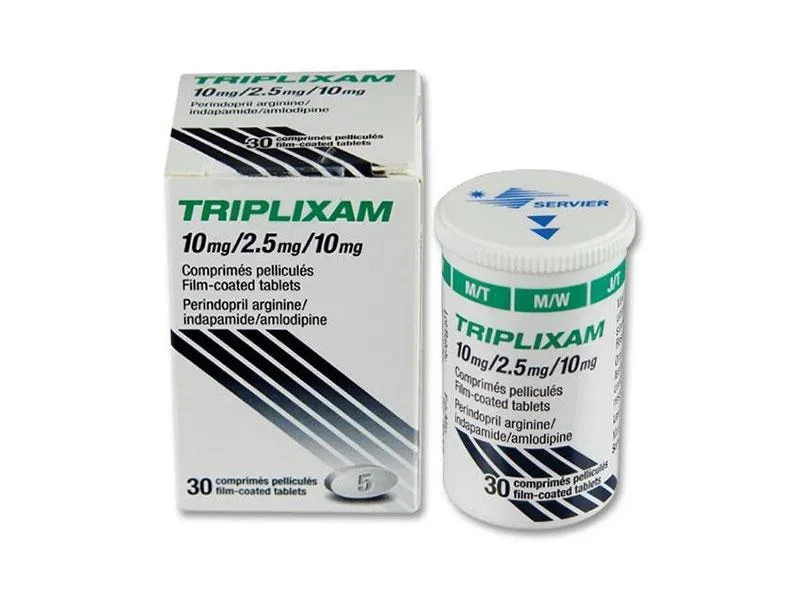
Triplixam (Perindopril Arginine, Indapamide, Amlodipine) Coated Tablets 10 mg/2.5 mg/10 mg. №30 Vial
Ingredients:
- Perindopril arginine: 10 mg
- Indapamide: 2.5 mg
- Amlodipine: 10 mg
Dosage:
The recommended dose is one tablet daily. It is important to follow the instructions of your healthcare provider.
Indications:
Triplixam tablets are indicated for the treatment of hypertension. This combination medication helps to control blood pressure effectively.
Contraindications:
Do not use Triplixam if you are allergic to any of the components or if you have a history of angioedema related to previous ACE inhibitor therapy.
Directions:
Take one tablet daily with water, preferably at the same time each day. It can be taken with or without food.
Scientific Evidence:
- Triplixam has shown significant efficacy in lowering blood pressure levels in patients with hypertension. Studies have demonstrated that the combination of perindopril, indapamide, and amlodipine provides better control of blood pressure compared to individual components alone.
- Clinical trials have also highlighted the cardiovascular benefits of Triplixam, including reducing the risk of stroke and heart attacks. The synergistic effects of the three active ingredients contribute to improved outcomes in hypertensive patients.
Additional Information:
- It is essential to regularly monitor blood pressure levels while taking Triplixam to ensure optimal control. Inform your healthcare provider about any side effects or concerns during treatment.
- Consult your doctor before starting or changing any medication regimen, especially if you have underlying medical conditions or are pregnant or breastfeeding.