Description
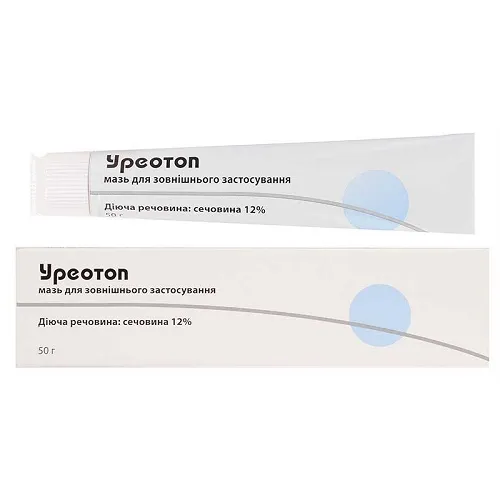
Ureotop (Urea) Ointment 12% 50 g Tube
Ingredients:
- Active Ingredient: 12% urea
- Inactive Ingredients: water, mineral oil, cetyl alcohol, glyceryl monostearate, petrolatum, propylene glycol, sorbitan stearate
Dosage:
Usage: Apply a thin layer 1-3 times daily to affected area
Indications:
Ureotop ointment is for dry, rough, and scaly skin conditions like psoriasis, eczema, and ichthyosis.
Contraindications:
Avoid: Allergy to ingredients, open wounds, broken skin. Consult if pregnant or breastfeeding.
Directions:
Preparation: Clean and dry area before application
Application: Massage gently until absorbed. Wash hands after, unless treating hands.
Scientific Evidence:
Research supports urea’s hydrating and keratolytic properties, improving skin hydration and reducing scaling.
Additional Information:
- Long-term Use: Well-tolerated
- Sun Sensitivity: Use sunscreen and protective clothing
- Discontinue: If irritation occurs
Pharmacological Effects: Urea increases skin water content, softens and dissolves outer skin layer substances, hydrating skin and promoting dead skin cell shedding.
Clinical Trials: Studies show urea ointments improve xerosis and hyperkeratosis, enhancing skin hydration and reducing itching in dry skin conditions.