Description
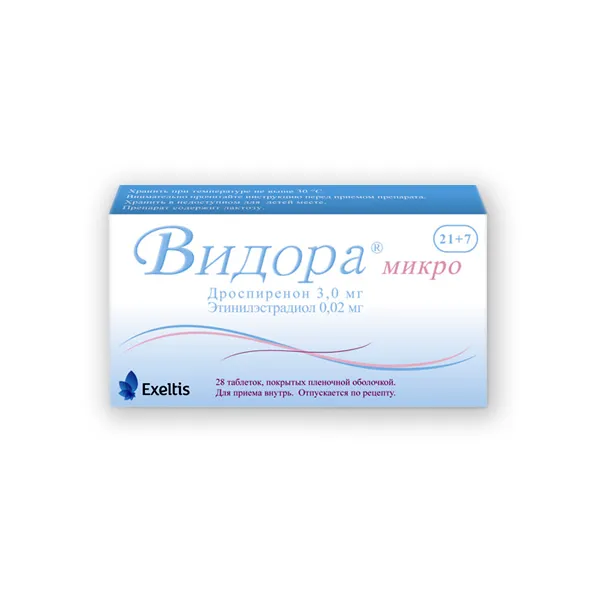
Vidora Micro (Drospirenone, Ethinylestradiol) Coated Tablets 3 mg/0.02 mg. №28
Ingredients
Each coated tablet contains 3 mg of drospirenone and 0.02 mg of ethinylestradiol.
Dosage
The recommended dosage is one tablet daily for 28 days, starting on the first day of the menstrual cycle.
Indications
Vidora Micro tablets are indicated for the prevention of pregnancy. They are also used to treat symptoms of premenstrual dysphoric disorder (PMDD) and moderate acne in women seeking contraception.
Contraindications
Do not use Vidora Micro if you are pregnant or breastfeeding. It is contraindicated in women with a history of blood clots, certain cancers, liver disease, or uncontrolled high blood pressure.
Directions
Take one tablet daily at the same time each day with or without food. Follow the instructions on the package for the correct use of the product.
Scientific Evidence
Studies have shown that the combination of drospirenone and ethinylestradiol in Vidora Micro tablets effectively prevents pregnancy by suppressing ovulation, thickening cervical mucus, and altering the endometrium. The tablets have also been found to reduce the severity of PMDD symptoms and improve acne in women.
- One study published in the Journal of Contraception reported that Vidora Micro was well-tolerated and had a high contraceptive efficacy rate.
- The research highlighted the positive impact of the medication on menstrual cycle regularity and hormonal balance.
Additional Information
- Storage: Store Vidora Micro tablets at room temperature away from moisture and heat.
- Side Effects: Common side effects may include nausea, headache, breast tenderness, and breakthrough bleeding. Contact your healthcare provider if you experience severe side effects.
- Interactions: Inform your doctor about all medications and supplements you are taking before starting Vidora Micro to avoid potential drug interactions.