Description
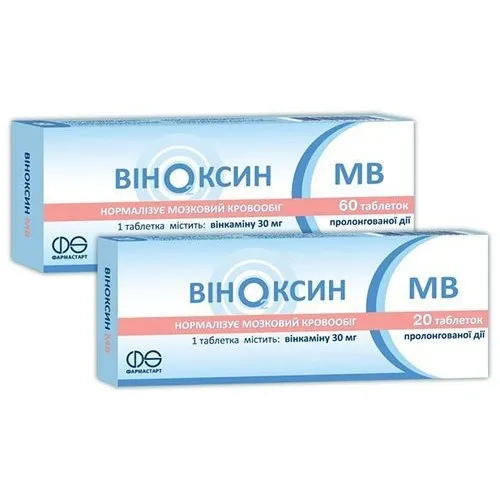
Vinoxin MV (vincamine) Tablets with Prolonged Release 30 mg. №60
Ingredients
- Active ingredient: Vincamine 30 mg.
- Other ingredients: Microcrystalline cellulose, lactose monohydrate, hydroxypropyl methylcellulose, magnesium stearate, talc.
Dosage
Recommended dosage: 1 tablet daily, or as directed by a healthcare professional. Do not exceed the recommended dose.
Indications
Vinoxin MV tablets are indicated for the improvement of cerebral blood flow and cognitive function. They are commonly used in managing symptoms associated with cerebrovascular disorders.
Contraindications
Do not use Vinoxin MV tablets if you are:
- Pregnant or breastfeeding
- Allergic to any of the ingredients
- Suffering from severe cardiac arrhythmias
Directions
Take Vinoxin MV tablets orally with water, preferably with a meal to reduce gastrointestinal side effects. Do not crush or chew the tablets. Consult a healthcare professional for personalized dosing instructions.
Scientific Evidence
Vincamine, the active ingredient in Vinoxin MV tablets, has been extensively studied for its effects on cerebral blood flow and cognitive function. Research has shown that vincamine can enhance cerebral circulation, potentially improving memory and cognitive performance in individuals with cerebrovascular insufficiency.
Additional Information
Vinoxin MV tablets with prolonged release offer a convenient once-daily dosing regimen, ensuring sustained therapeutic levels of vincamine in the body. Individual responses to the medication may vary, and it is recommended to consult a healthcare provider before starting any new supplement regimen.
Pharmacological Effects: Vincamine acts as a cerebral vasodilator, increasing blood flow to the brain and enhancing oxygen and glucose utilization. This mechanism of action contributes to improved cognitive function and may benefit individuals with conditions affecting cerebral circulation.
Clinical Trials: Studies have demonstrated the efficacy of vincamine in improving symptoms associated with cerebrovascular disorders. Significant improvements in cognitive function and quality of life have been reported in patients receiving vincamine treatment compared to placebo.