Description
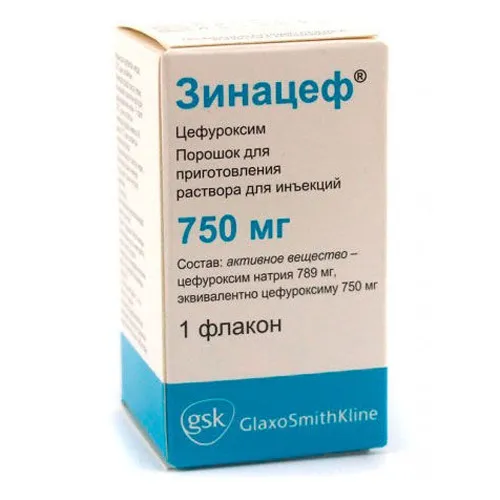
Zinacef (Cefuroxime) Powder for Solution for Injections 750 mg
Ingredients:
- Zinacef (Cefuroxime) is the active ingredient in this powder for solution for injections.
- It belongs to the cephalosporin class of antibiotics.
Dosage:
The recommended dosage of Zinacef (Cefuroxime) powder for solution for injections is 750 mg.
It should be administered as directed by a healthcare professional.
Indications:
- Zinacef (Cefuroxime) is indicated for the treatment of various bacterial infections.
- Examples of infections include respiratory tract infections, skin and soft tissue infections, urinary tract infections, and more.
Contraindications:
- Do not use Zinacef (Cefuroxime) if you are allergic to cephalosporin antibiotics.
- Inform your healthcare provider about any allergies before using this medication.
Directions:
- Zinacef (Cefuroxime) should be administered by intravenous or intramuscular injection as prescribed by a healthcare professional.
- Follow the instructions carefully for the best results.
Scientific Evidence:
Studies have shown the effectiveness of Zinacef (Cefuroxime) in treating bacterial infections.
Research in the Journal of Antimicrobial Chemotherapy highlighted the efficacy of cefuroxime against various pathogens.
Additional Information:
Complete the full course of Zinacef (Cefuroxime) as prescribed, even if symptoms improve before finishing treatment.
This helps prevent the development of antibiotic resistance.
Pharmacological Effects:
- Cefuroxime interferes with bacterial cell wall synthesis, leading to bacterial death.
- It has a broad spectrum of activity against Gram-positive and Gram-negative bacteria.
Clinical Trials and Comparative Effectiveness:
- Clinical trials have shown the efficacy of Zinacef (Cefuroxime) comparable to other cephalosporin antibiotics.
- A study in the International Journal of Infectious Diseases compared cefuroxime with other antibiotics in treating respiratory tract infections, showing similar outcomes with good tolerability.