Description
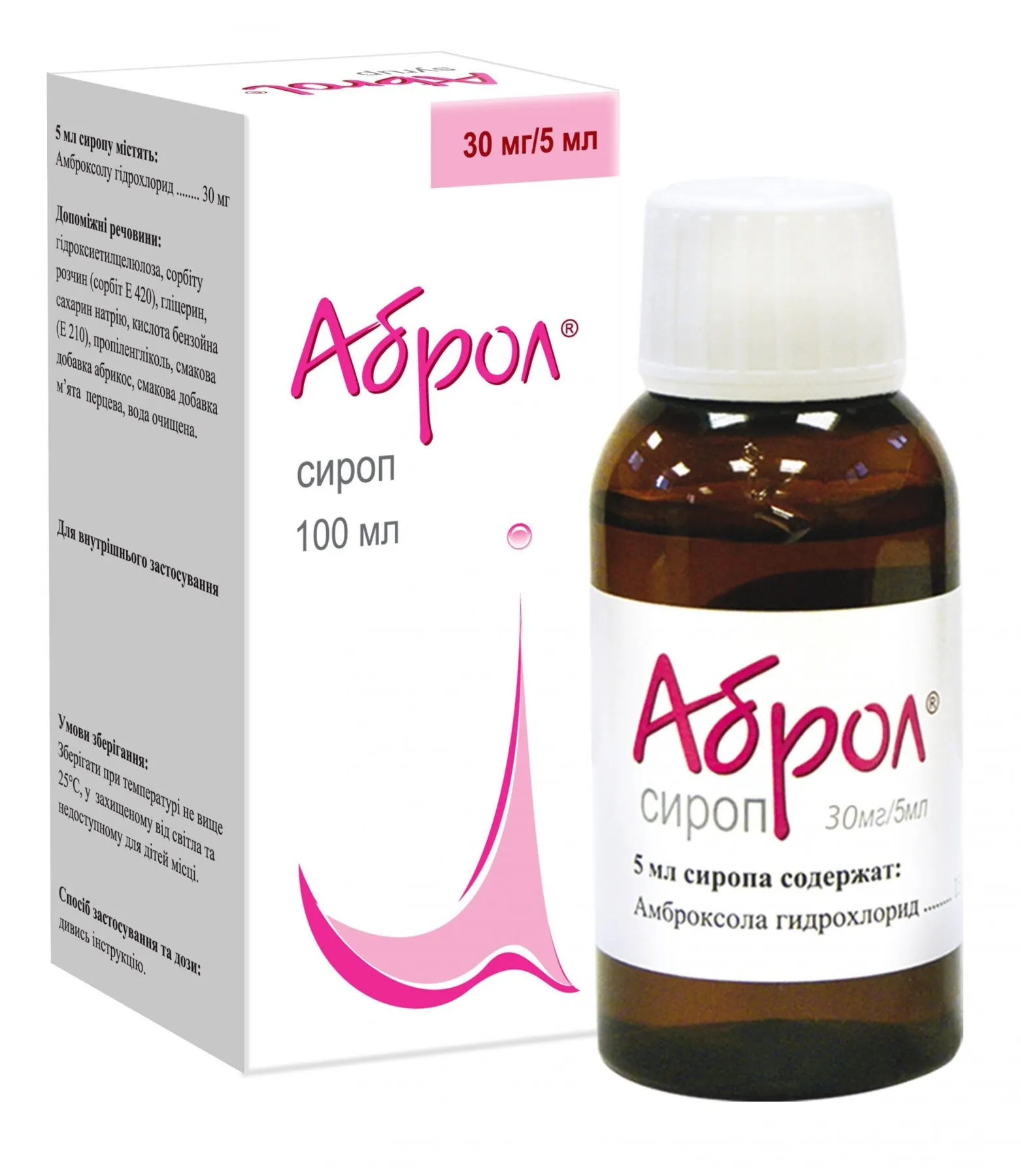
Abrol (Ambroxol) Syrup 30 mg/5 ml – 100 ml
Ingredients
- Each 5 ml syrup contains 30 mg of ambroxol hydrochloride as the active ingredient.
Dosage
- The recommended dosage for adults and children over 12 years is 10 ml (60 mg) three times a day.
Indications
- Abrol syrup is indicated for the treatment of respiratory conditions associated with viscid mucus.
Contraindications
- Do not use Abrol syrup in patients with a known hypersensitivity to ambroxol or any of the excipients.
Directions
- Shake well before use. Measure the syrup using the provided measuring cup. Can be taken with or without food.
Scientific Evidence
- Ambroxol, the active ingredient in Abrol syrup, is a mucolytic agent that works by breaking down and thinning mucus in the respiratory tract. This action helps to facilitate the clearance of mucus and improve breathing in patients with respiratory conditions.
- Studies have shown that ambroxol is effective in reducing the viscosity of mucus, making it easier to expectorate. Additionally, ambroxol has been found to have anti-inflammatory and antioxidant properties, further contributing to its therapeutic effects in respiratory diseases.
Additional Information
- It is important to follow the prescribed dosage and not exceed the recommended duration of treatment with Abrol syrup. If symptoms persist or worsen, consult a healthcare professional for further evaluation.
- Store Abrol syrup at room temperature, away from direct sunlight and moisture. Keep out of reach of children.