Description
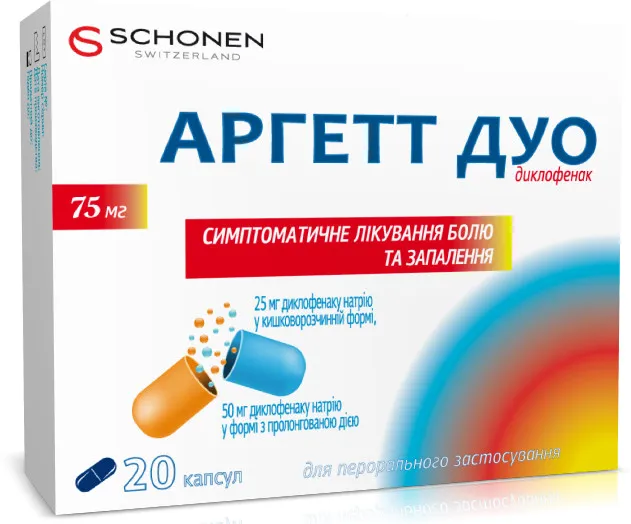
Argett Duo (Diclofenac) Capsules with Modified Release 75 mg. №20
Composition
Each capsule contains 75 mg of diclofenac with modified release.
Mechanism of Action
Diclofenac, the active ingredient in Argett Duo, acts by inhibiting the production of prostaglandins, which are mediators of pain and inflammation in the body.
Pharmacological Properties
The modified release formulation of Argett Duo ensures a sustained and prolonged release of diclofenac, providing consistent relief from pain and inflammation.
Indications for Use
Argett Duo capsules are indicated for the relief of pain and inflammation in conditions such as arthritis, ankylosing spondylitis, and acute gout.
Contraindications
Avoid the use of Argett Duo if you have a history of allergic reactions to diclofenac or other NSAIDs, or if you have active peptic ulcers.
Side Effects
Potential side effects of Argett Duo include gastrointestinal ulcers and cardiovascular events. It is important to adhere to the prescribed dosage and treatment duration to minimize these risks.
Usage Instructions
Take one capsule of Argett Duo daily with food. Swallow the capsule whole with a full glass of water; do not crush or chew it.
Benefits Compared to Analogues
Clinical trials have shown that Argett Duo offers effective pain management with a lower risk of gastrointestinal side effects compared to immediate-release diclofenac formulations. This makes Argett Duo a preferred option for long-term pain management.
Suitable Patient Groups
Argett Duo is suitable for adults seeking relief from pain and inflammation associated with various musculoskeletal conditions. Consult a healthcare provider before use, especially if you have a history of heart disease or gastrointestinal issues.
Storage and Shelf Life
Store Argett Duo in a cool, dry place away from direct sunlight. Check the expiry date on the packaging and do not use the product after the specified date.
Packaging Description
Argett Duo is available in packs containing 20 capsules of 75 mg each, with modified release properties.
Clinical Evidence and Proven Effectiveness
Studies have demonstrated the efficacy of Argett Duo in managing chronic pain conditions by providing sustained pain relief with reduced gastrointestinal side effects. The modified release formulation ensures a steady release of diclofenac, enhancing its therapeutic benefits.