Description
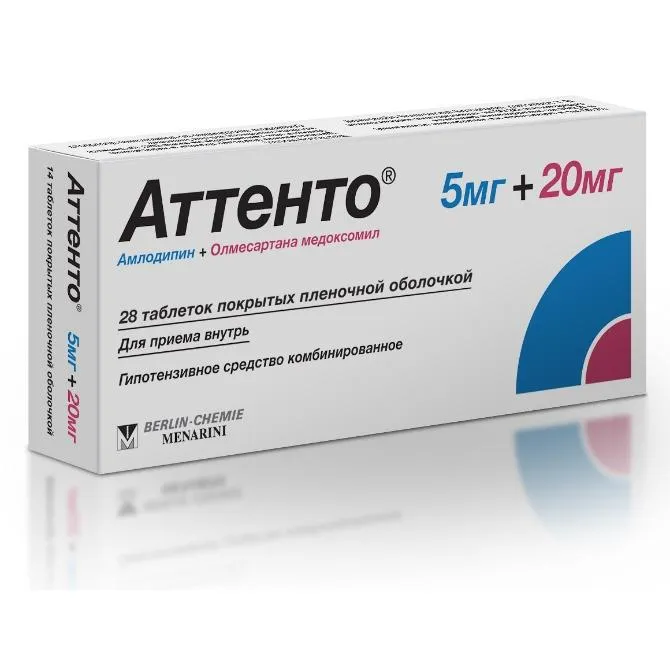
Attento (Olmesartan Medoxomil) Coated Tablets 20 mg/5 mg. №28
Ingredients:
Each tablet contains 20 mg of olmesartan medoxomil and 5 mg of an additional coating.
Mechanism of Action:
Olmesartan medoxomil acts as an angiotensin II receptor antagonist, selectively blocking the binding of angiotensin II to its receptors in vascular smooth muscle and adrenal gland. By inhibiting this interaction, olmesartan medoxomil prevents vasoconstriction and the release of aldosterone, leading to vasodilation and decreased blood pressure.
Pharmacological Properties:
Olmesartan medoxomil is a prodrug that is rapidly converted to the active metabolite olmesartan during absorption from the gastrointestinal tract. The active metabolite selectively inhibits the angiotensin II type 1 receptor, exerting its antihypertensive effects.
Indications for Use:
Attento tablets are indicated for the treatment of hypertension to lower blood pressure in adult patients.
Contraindications:
Attento should not be used in pregnant women, patients with severe renal impairment, or individuals with a history of angioedema related to previous ACE inhibitor therapy.
Side Effects:
Common side effects of Attento may include dizziness, headache, and fatigue. Patients should be monitored for signs of renal impairment, although this side effect is rare. If any serious adverse reactions occur, immediate medical attention is necessary.
Usage Instructions:
The recommended dosage of Attento is one tablet daily, to be taken orally with water. The tablet can be administered with or without food at the same time each day, following the guidance of a healthcare professional.
Benefits Compared to Analogues:
Attento, containing olmesartan medoxomil, has demonstrated superior efficacy in lowering both systolic and diastolic blood pressure compared to other angiotensin II receptor blockers. Its mechanism of action provides effective blood pressure control with a favorable tolerability profile.
Suitable Patient Groups:
Attento is suitable for adult patients with hypertension. Special care should be taken in elderly patients and those with mild to moderate renal impairment. The safety and efficacy of Attento in pediatric populations have not been established.
Storage Conditions and Shelf Life:
Attento should be stored at room temperature away from moisture and heat. The shelf life of the product is as indicated on the packaging.
Packaging Description:
Attento is available in packages containing 28 coated tablets. The packaging should be kept intact and stored in a secure place out of reach of children.
Scientific Evidence:
Studies have shown that olmesartan medoxomil effectively lowers blood pressure by blocking the vasoconstrictor and aldosterone-secreting effects of angiotensin II. Clinical research published in the Journal of Clinical Hypertension has demonstrated the superior efficacy of olmesartan medoxomil in reducing blood pressure levels compared to other antihypertensive agents.